
Kiora Pharmaceuticals (Nasdaq: KPRX) develops new medicines to help people with eye diseases see better. Their goal is to improve vision and eye health for patients.
Kiora's name comes from a Maori phrase meaning "have life" or "be healthy." The company name reflects its mission to help give the gift of better vision and eye health to more people.
With a refreshed leadership team and a revolutionary small molecule, KIO-301, Kiora Pharmaceuticals aims to make a positive difference for patients struggling with vision problems.
Nasdaq: KPRX
IR Website: https://ir.kiorapharma.com
Nasdaq Profile: https://www.nasdaq.com/market-activity/stocks/kprx
Headquarters: Encinitas, California
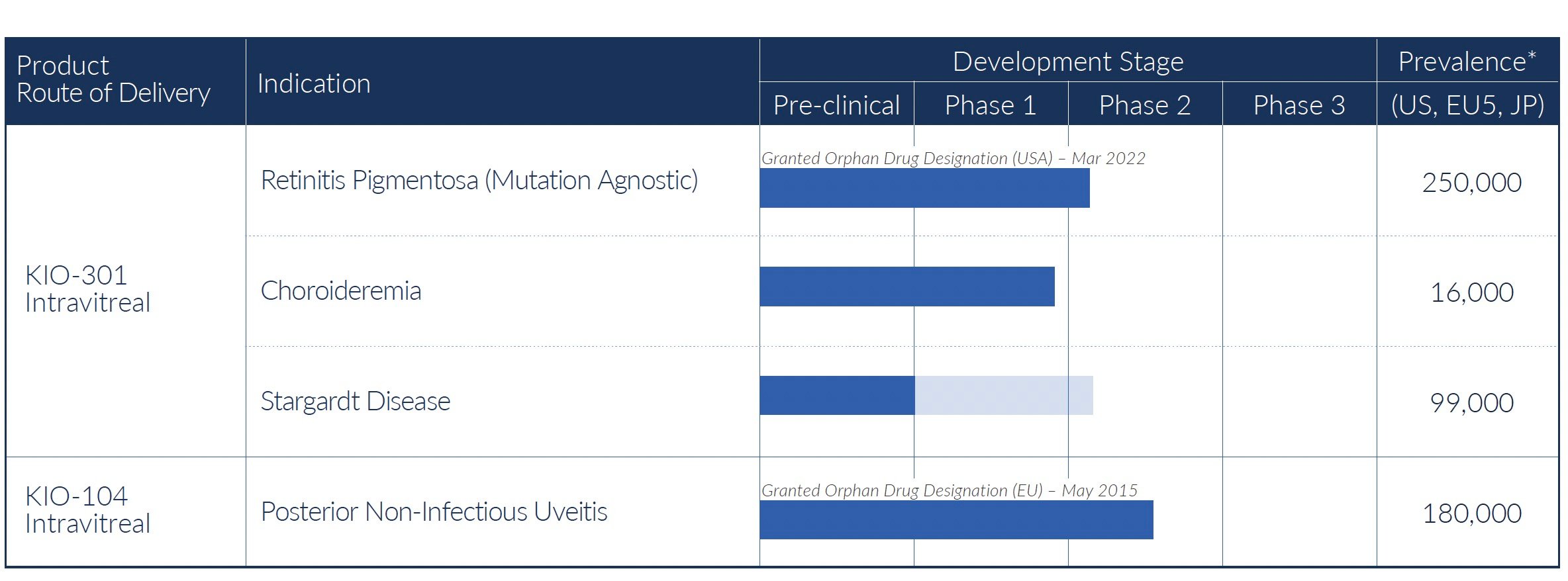
Kiora Pharmaceuticals emerged in November 2021 as part of a major strategic shift in its clinical development strategy under the direction of its new CEO, Brian Strem, Ph.D., and the acquisition of Bayon Therapeutics, the company he co-founded with Kiora’s Chief Development Officer, Eric Daniels, M.D., to develop KIO-301. Today, the company is focused on the treatment of rare vision disorders caused by damage to the retina. KIO-301, its priority pipeline asset, is a molecular photoswitch designed to restore light perception in patients with inherited or age-related retinal degeneration like Retinitis Pigmentosa. Kiora initiated a Phase 1b trial of KIO-301 in late 2022, with full results expected in Q4 2023. Based on early-reported findings, the Company expanded development to Choroideremia and Stargardt’s Disease, two additional inherited retinal diseases that could be addressed with a molecular photoswitch. If successful, the company believes KIO-301 could offer patients with certain rare eye diseases a revolutionary treatment option.
TALK TO MANAGEMENT
Kiora Pharmaceuticals is always available to talk to current and potential investors. They're happy to answer any questions you may have and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
All content on this page is accurate as of 11.18.23.
Kiora Pharmaceuticals At A Glance

Kiora Pharmaceuticals is a clinical-stage biotech company developing novel therapies for rare, orphan retinal diseases.
The company's lead asset, KIO-301, is a first-in-class retinal therapy designed to restore lost vision in patients with degenerative retinal conditions like Retinitis Pigmentosa, Choroideremia, and Stargardt's Disease. KIO-301 works by reactivating viable retinal ganglion cells after photoreceptors have died.
Kiora is also developing KIO-104, a small molecule inhibitor of DHODH, for the treatment of non-infectious uveitis, a rare inflammatory eye disease.
The company has additional pipeline assets, including KIO-101 for ocular inflammation and KIO-201 for corneal defects and injuries, for which it is seeking strategic partnerships to support further development.
Early clinical results demonstrate improved light perception in RP patients treated with KIO-301, supporting the company's plans to advance development. This novel dual mechanism gives KIO-301 and KIO-104 potential as approved medicines versus just slowing disease progression.
Kiora's experienced management team has proven drug development and commercialization expertise in ophthalmology. The company is focused on executing its clinical strategy while maintaining a financial runway.
Key Considerations:
Novel approach unlike any current medicines
KIO-301 works differently than other vision medicines by making cells light-sensitive after photoreceptors die. It is not a gene therapy. Instead, it targets downstream neurons, so it may help RP patients regardless of genetic cause. This unique Mechanism of Action (MOA) gives hope of visual restoration instead of slowing vision loss.
Orphan drug designation for RP in the US
KIO-301 received orphan drug designation from the FDA for treating RP. This gives development incentives and exclusivity benefits if approved. It recognizes the high unmet need in RP, as there are no approved treatments to restore vision. Kiora intends to apply for similar designations in other countries.
Phase 1 safety data and evidence of biological activity
A Phase 1 study showed KIO-301 was safe when injected in the eye. Patients also had measurable improvements in light perception tests, thus indicating biological activity. One completely blind patient could see his wife’s mobile phone turn on and off after treatment. These early results support the drug's mechanism of action.
Kiora Adds Focus to KIO-104 for Rare Eye Disease
Kiora Pharmaceuticals is advancing the development of KIO-104 for the treatment of non-infectious posterior uveitis, a rare inflammatory eye disease that can lead to vision loss. KIO-104 is a novel small molecule inhibitor of DHODH, an enzyme involved in inflammation. By suppressing overactive immune cells, KIO-104 may provide a new non-steroidal treatment approach for uveitis.
Seasoned leadership team
Kiora has an experienced management team led by CEO Brian Strem, who has successfully developed other ophthalmic drugs. The clinical team includes experts in retinal diseases, while the CMC team has drug manufacturing expertise. This knowledgeable team increases the chance of successful development.
Press Releases & Media Coverage
Investor Presentation


To download the Kiora Pharmaceuticals investor presentation, please fill out the form below.
Stock Chart (Intraday)
Stock Chart (Historical)
Stock Detail
A Video Overview of Kiora Pharmaceuticals

Learn more about Kiora Pharmaceuticals in this 90-second video overview.
Source: Company documents
Kiora Pharmaceuticals Focuses on Treatments for Rare Blinding Conditions

Blindness can have a devastating impact on a person's quality of life. Loss of vision robs individuals of their independence and prevents them from fully participating in work, school, social activities, and everyday tasks. For people afflicted with rare inherited retinal diseases that lead to blindness, the emotional toll can be immense. A study published in JAMA Ophthalmology ranked losing eyesight as the condition with the greatest effect on day-to-day life. Kiora Pharmaceuticals is a clinical-stage biotechnology company developing novel therapies for these overlooked patient populations.
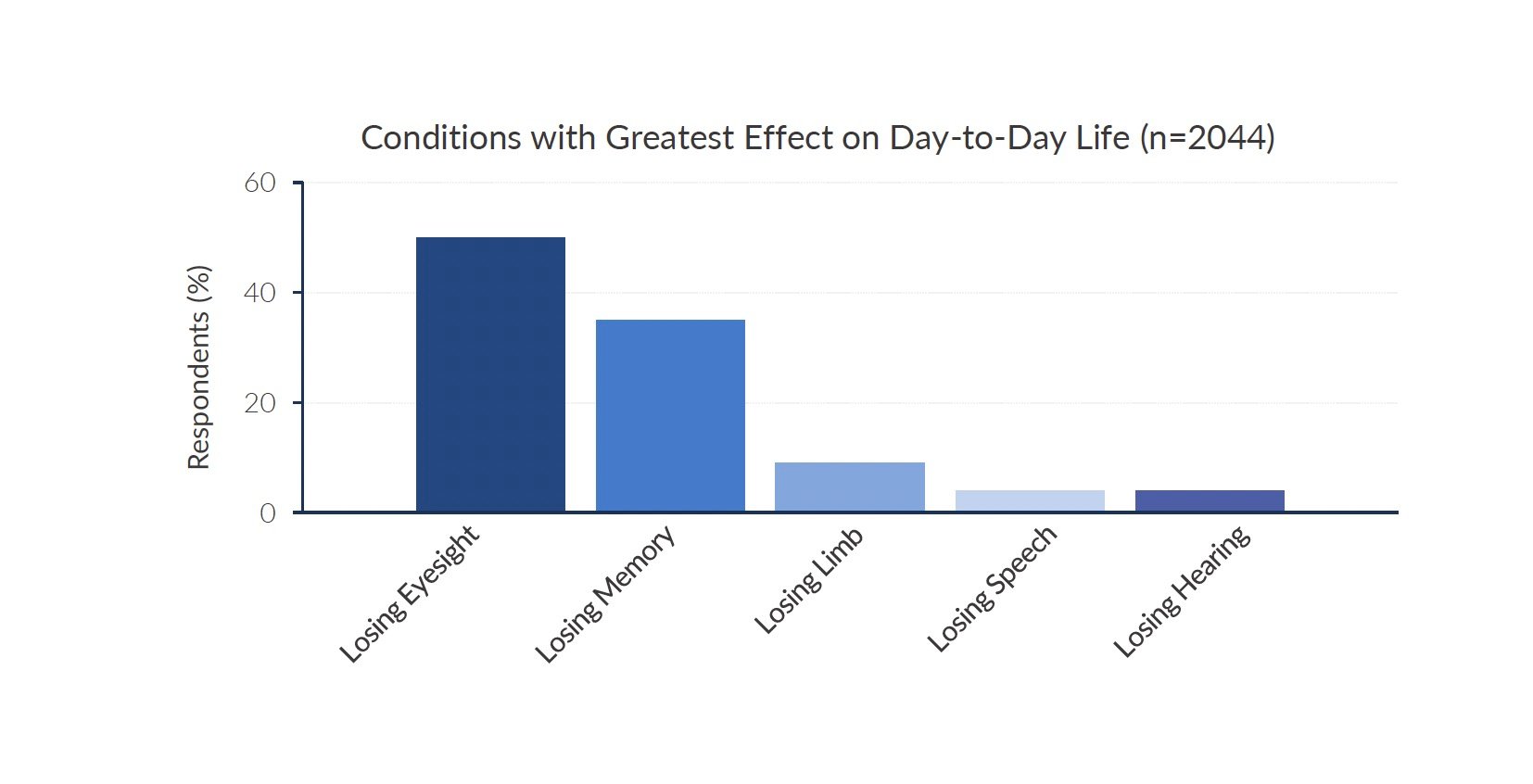
Source: Company documents
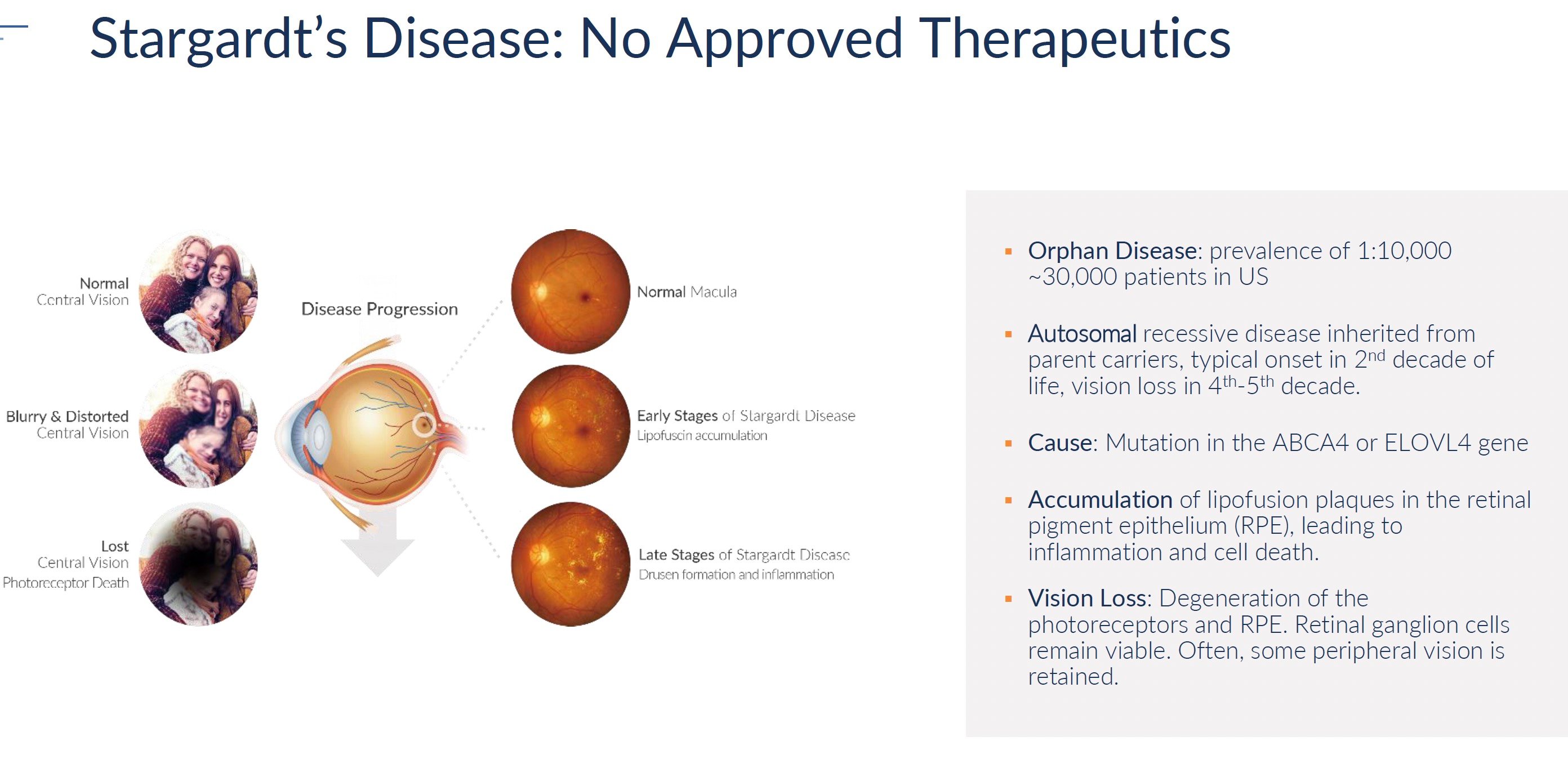
Retinitis pigmentosa, choroideremia, and Stargardt's disease are rare genetic disorders that cause progressive vision loss and eventual blindness. Collectively they impact approximately 150,000 patients in the United States. The rarity of these conditions means that drug development has largely ignored them. No treatments exist to slow or reverse the course of vision decline. At best, patients are offered visual aids and assistive devices to maximize remaining functional vision. However, the underlying disease continues to destroy photoreceptors and retinal pigment epithelium, leading to complete blindness typically by middle age.

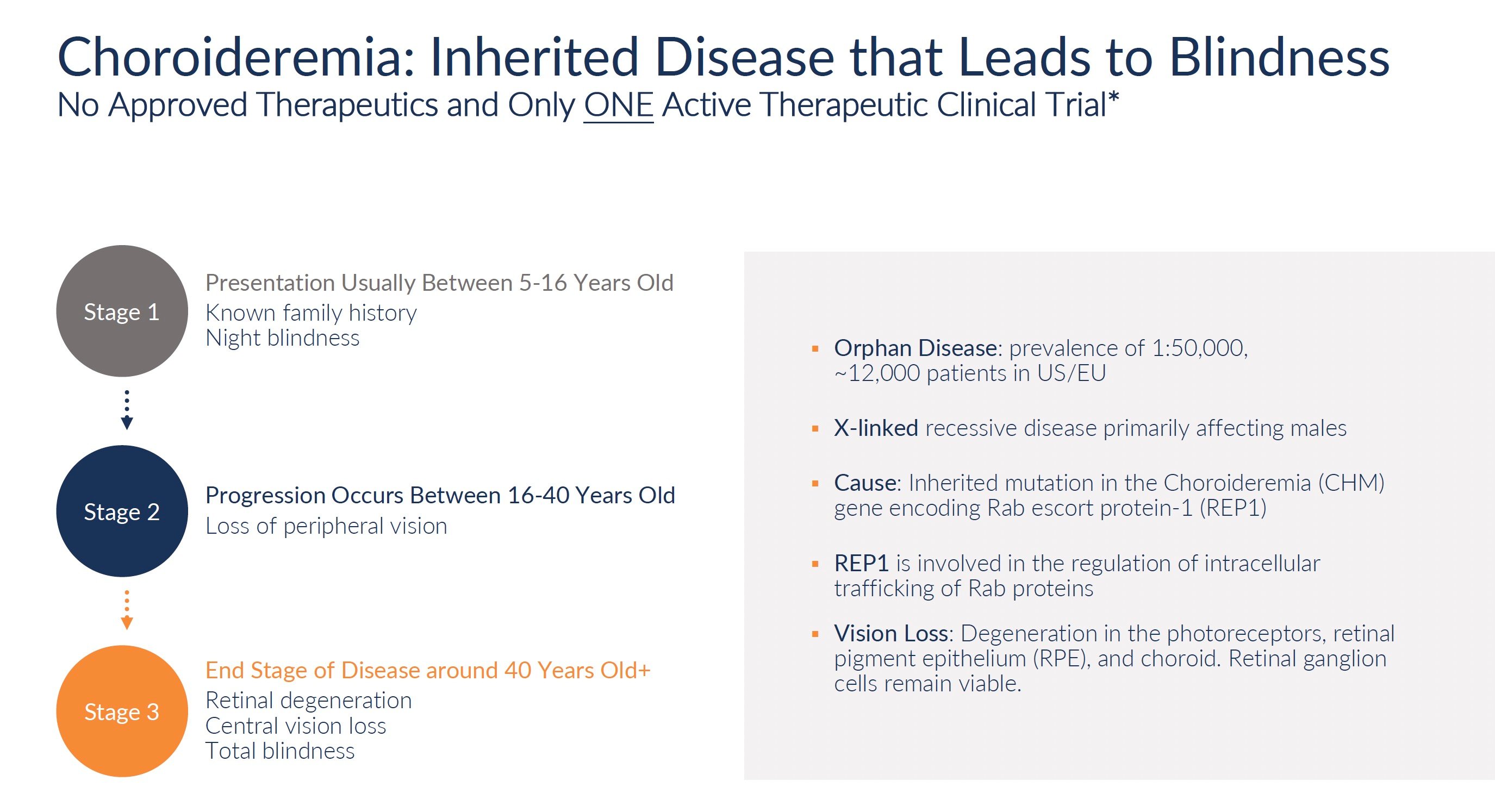
Source: Company documents
Kiora aims to change the status quo for people with inherited retinal dystrophies. Through an improved understanding of disease pathology, the company has identified a novel opportunity to reawaken dormant retinal ganglion cells using its investigational therapy KIO-301. Retinal ganglion cells are normally activated by photoreceptors to transmit visual signals to the brain. When photoreceptors die, retinal ganglion cells go dormant but remain viable. KIO-301 enters retinal ganglion cells and essentially turns them “on” using light, thereby restoring some visual sensation even in the absence of photoreceptors.
Early-phase clinical results for KIO-301 in retinitis pigmentosa patients are promising. Participants experienced gains in light sensitivity, visual acuity, and functional vision based on various clinical tests. These benefits translated into meaningful improvements in quality of life and ability to perform daily activities. Patients reported exciting outcomes like identifying lights, motion, and shapes that were previously impossible to detect in their blindness.
While full data analysis is still underway, initial findings compel Kiora to advance KIO-301 for retinitis pigmentosa as well as expand development into related orphan diseases. The company plans to initiate phase 2 trials in choroideremia and Stargardt’s disease, two conditions sharing a similar pathogenesis of photoreceptor loss with intact inner retinal neurons. Strategic partnerships with patient advocacy groups will support trial enrollment and protocol design.
Kiora’s pipeline also includes KIO-104 for treatment of non-infectious uveitis, a rare inflammatory eye disease that often involves the retina. Uveitis has limited treatment options and a high rate of blindness if left uncontrolled. In early studies, KIO-104 utilizes a novel anti-inflammatory mechanism of action to reduce ocular inflammation and improve visual outcomes after just a single intravitreal injection. This has the potential to significantly improve the quality of life for uveitis patients by decreasing reliance on frequent steroid eye injections.
Guiding Kiora’s programs is a patient-first mentality and sense of urgency. For people progressing towards inevitable blindness, time is a precious commodity. Kiora aims to rapidly advance the development of KIO-301 and KIO-104 to address important unmet needs in multiple orphan retinal diseases. The company’s patient-centered approach is enabled by strategic industry partnerships, experienced leadership, and guidance from key opinion leaders in ophthalmology.
Kiora’s focus stands apart in an industry that often overlooks orphan diseases in favor of larger markets. Driven by patient perspectives, physician needs, and societal obligations, the company challenges the status quo. Kiora’s therapies in development represent hope for improved quality of life and preservation of functional vision in patient populations running out of time. The company’s passion and innovation reflect the transformative potential of modern ophthalmic drug development. By concentrating efforts on overlooked rare diseases, Kiora strives to restore both sight and dignity to people who are blind.
KIO-301 Is a Potential Vision-Restoring Medicine for Certain Incurable Eye Diseases

Retinitis Pigmentosa (RP) is a rare genetic eye disease that causes cells in the retina called photoreceptors to die. The condition is caused by mutations in over 150 different genes and affects approximately one out of every 3,000 to 5,000 people. In the United States alone, there are approximately 80,000 patients living with the disease.
Photoreceptors are special light-sensing cells that normally detect light and send signals to the brain so we can see.
When photoreceptors die in RP, other cells in the retina called retinal ganglion cells (RGCs) are still alive but can't sense light anymore. This leaves patients blind.
KIO-301 (benzyl ethyl aminoazobenzene quaternary ammonium) is a drug designed to enter into the RGCs and make them able to sense light again, even though the photoreceptors are dead.
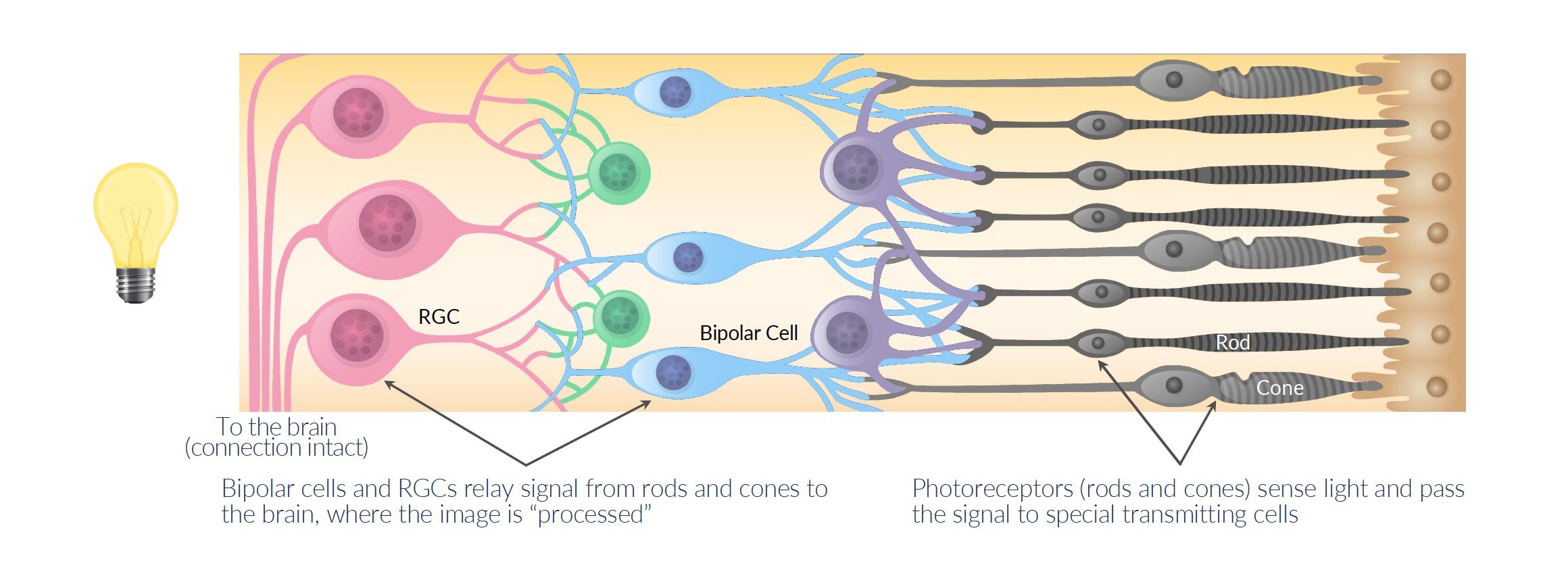
Source: Company Documents
KIO-301 works by attaching to specific sites called P2X7 that are only found on RGCs and other retinal cells. KIO-301 does not attach to P2X7 but rather simply gains entry into the cell via this upregulated protein. It is here where shape changing causes these neurons to signal the brain as to the presence/absence of light.
When light hits the eye, KIO-301 changes shape and blocks ion channels on the RGCs that normally let ions flow in and out.
Blocking these ion channels causes the RGCs to activate and send signals to the brain that light is detected, even without photoreceptors.
In summary, KIO-301 acts like a light switch to turn RGCs into light-sensing cells after photoreceptors die in RP. This is how it may be able to restore some degree of vision in patients with these retinal diseases.
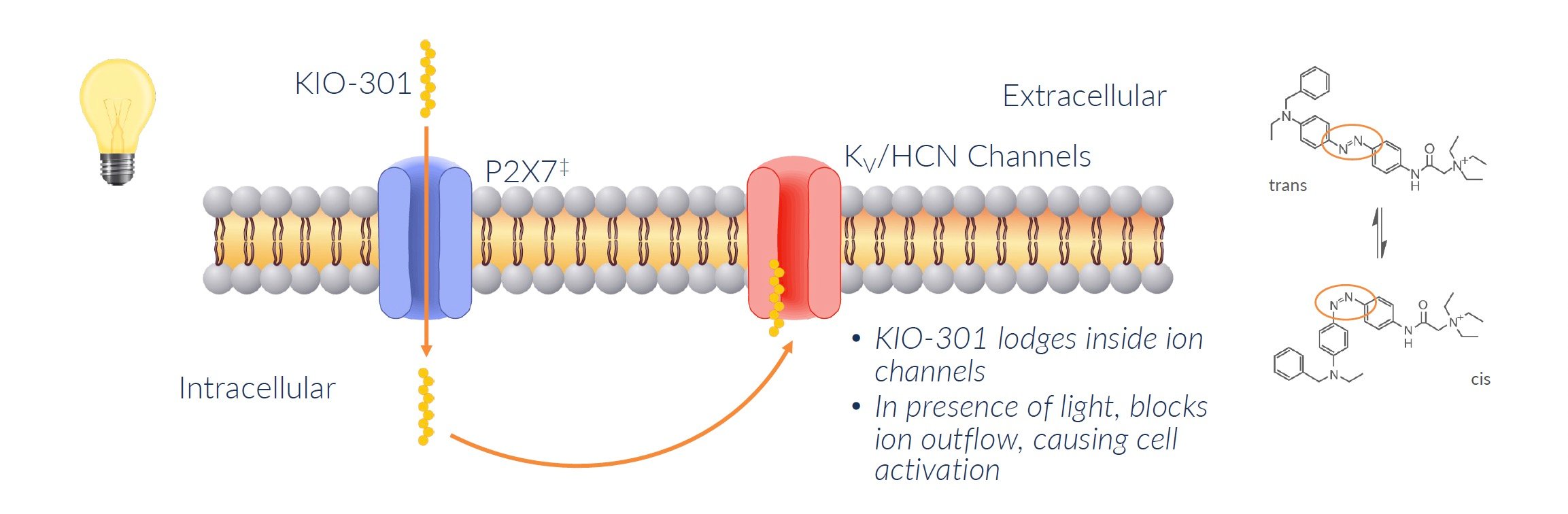
Source: Company Documents
KIO-301 Uses a Novel Mechanism

Most vision drugs try to prevent damage to photoreceptors or slow their loss in retinal diseases like retinitis pigmentosa (RP). But eventually, the photoreceptors die. KIO-301 takes an entirely different approach. It doesn't target the damaged photoreceptors at all. Instead, it targets retinal ganglion cells (RGCs) downstream from the photoreceptors.
Even when photoreceptors are gone in RP, RGCs remain alive but unable to detect light independently. KIO-301 enters the RGCs and acts like a light switch to make them sense light. When light hits the eye, the drug undergoes a shape change that blocks ion channels on the RGCs, causing them to fire signals to the brain that light is detected. Indeed, a tiny percentage of RGCs are light-sensitive and are thought to be key regulators of our circadian rhythm.
So unlike other vision drugs, KIO-301 restores vision by making cells sense light again. This gives real hope of improving vision in RP, not just slowing decline. The novel approach means KIO-301 may help RP patients regardless of which genetic mutation caused their disease. It just flips the light switch back on in the remaining retinal cells.
This first-of-its-kind mechanism is why KIO-301 offers new hope to patients facing inevitable blindness from photoreceptor loss. No other vision drug can bring back light detection like KIO-301 aims to.
To watch a two-minute-long patient interview, please click below:
Source: Company documents
Orphan Drug Designation From The FDA

In March 2022, KIO-301 received orphan drug designation from the FDA for treating RP. This special status is given to encourage the development of medicines for rare diseases like RP that have high unmet medical needs.
There are currently no FDA-approved treatments that can restore lost vision in RP patients.
The orphan designation means KIO-301 will get certain benefits during development, like tax credits and waiver of FDA fees. The designation also provides market exclusivity if KIO-301 is approved, which means the FDA cannot approve other medicines for the same indication for seven years. The designation increases the commercial potential and incentives for Kiora to advance development.
Kiora plans to apply for similar orphan designations in other countries beyond the US.

Source: Company reports
Phase 1b Safety Data and Evidence of Biological Activity

The Phase 1b trial of KIO-301 in retinitis pigmentosa (RP) patients at two sites in Australia was a critical first step to see if this first-in-class treatment is safe when administered directly into the eye. Encouragingly, the results showed no concerning safety issues. This gives confidence that larger trials can now proceed and continue evaluating the optimal dose and dosing schedule.
Phase 1b was an open-label clinical trial to evaluate the safety and biological activity of KIO-301 in patients with retinitis pigmentosa (RP). There were 2 groups or cohorts of 6 patients each. Cohort 1 included RP patients with no light perception or bare light perception. Cohort 2 included RP patients with hand motion or count fingers vision. The study was open-label, meaning everyone got the active drug rather than a placebo.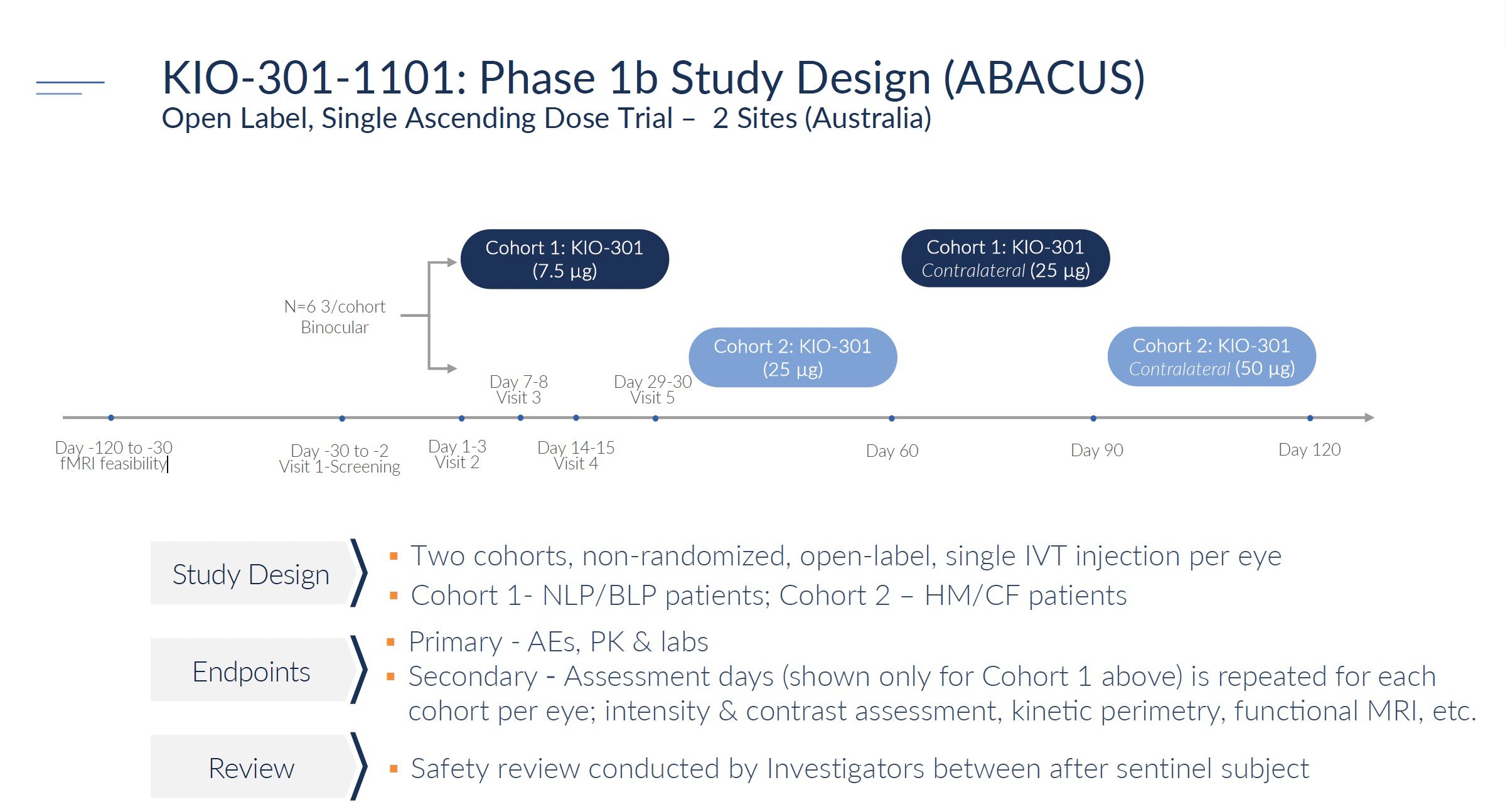
Source: Company data
Each patient received a single injection of KIO-301 into the eye (called intravitreal injection). Cohort 1 started at a low dose of 7.5 μg. Once deemed safe, the next Cohort 2 was tested at a higher 25 μg dose. Patients were followed for 90 days after the injection and had 5 study visits for safety and vision testing.
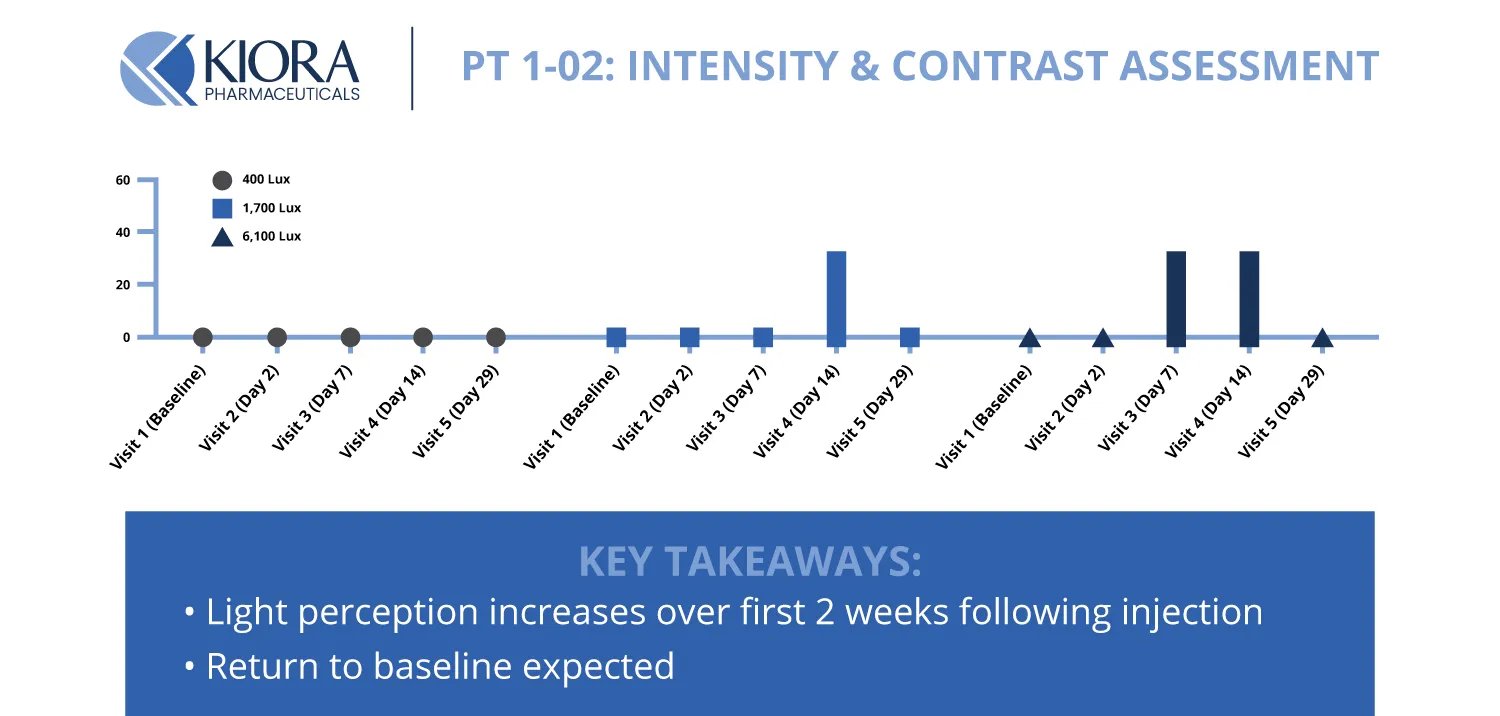
However, what was particularly notable in Phase 1 was that participants had measurable gains in visual function after treatment. One patient could correctly identify a 60-watt light bulb held up that they couldn't detect before - a tangible sign that KIO-301 was restoring light sensitivity. Overall, light perception increased over the first 2 weeks following injection.
Source: Company data
Other patients in the study showed an improved ability to detect lights at different intensities or contrasts. These vision enhancements indicate that the drug is indeed reaching viable retinal cells and converting them into light-sensing neurons as intended. Importantly, the results help validate that KIO-301 works via its novel mechanism of action - providing biological proof of concept.
Demonstrating the drug engages its target cells in humans and impacts vision is a major de-risking event. It significantly increases the probability that KIO-301 will ultimately prove effective in restoring vision lost to photoreceptor damage in diseases like RP.
While Phase 1 studies primarily assess safety, seeing these early signals that KIO-301 impacts biological function in patients provides confidence as the drug now moves into larger Phase 2 and 3 trials.
A full report on the data from Phase 1 was presented on November 4, 2023, at the American Academy of Ophthalmology annual conference (AAO 2023). Click the link to view the company's press release containing details of the results: AAO Late-Breaking: Kiora's Small Molecule Photoswitch Demonstrates Meaningful Vision Improvements in Blind Patients with Retinitis Pigmentosa.
Because the early results look promising, they are adding 3 more patients to the trial who have no light perception. By the end of 2023, Kiora plans to request permission from the FDA to start a larger phase 2 trial of KIO-301. This next trial will begin in early 2024 and include a control group for comparison. So far, the KIO-301 injections appear safe and able to restore some visual function in blind patients. Kiora now wants to test the drug in other inherited retinal diseases that have similar disease processes.
KIO-104: A Fresh Approach to Uveitis Management

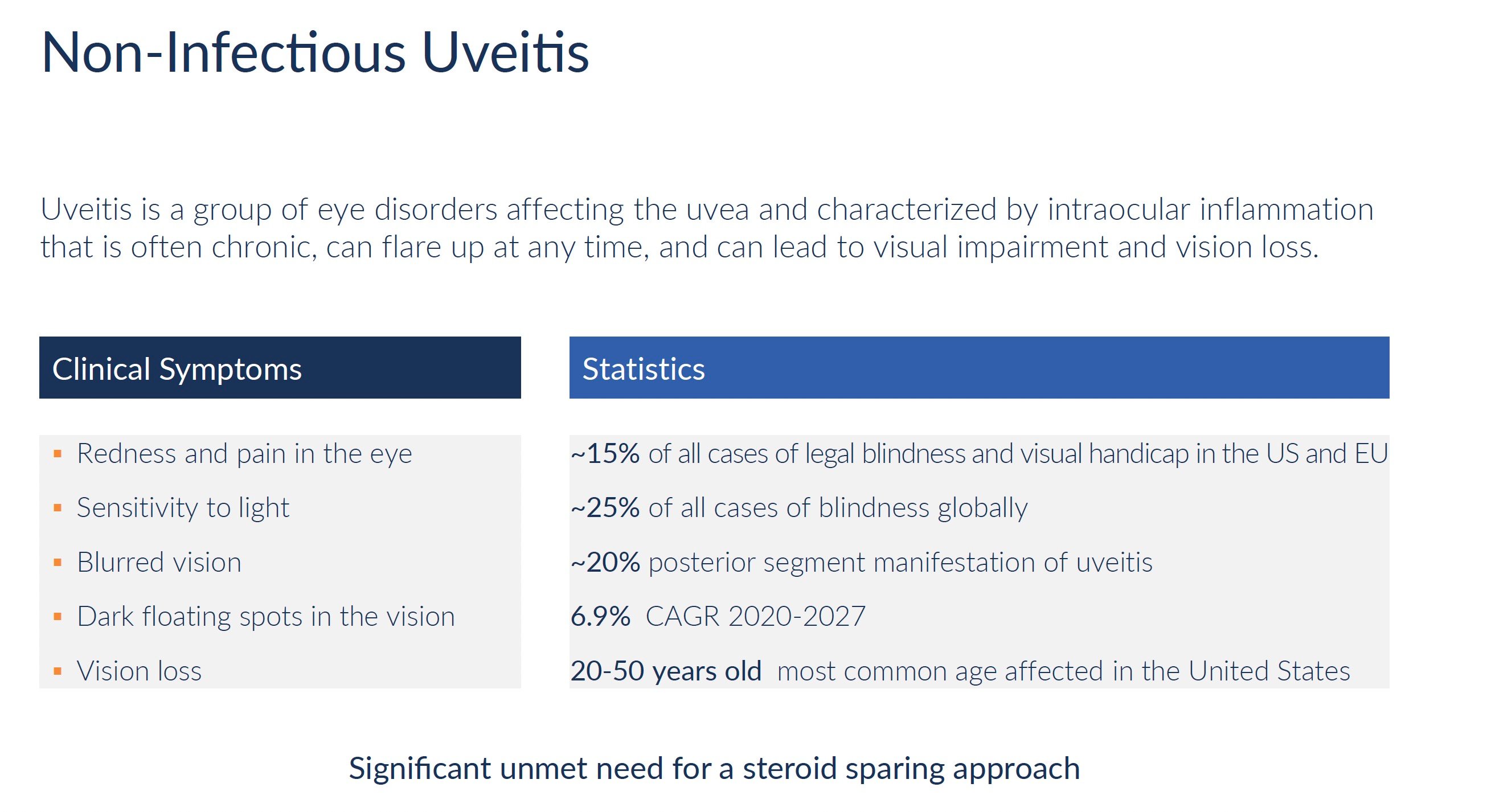
KIO-104 is an investigational drug designed to treat eye inflammation caused by non-infectious uveitis. Uveitis involves irritation and swelling within the eye that can permanently damage vision. Currently, uveitis is managed using steroid eye drops or injections. However, long-term steroid use can cause side effects.
KIO-104 works differently by inhibiting inflammation-causing immune cells and proteins. Early clinical trials show KIO-104 improves vision and reduces swelling inside the eye after a single injection. Patients tolerate the medication well with no serious side effects. KIO-104 has potential as a non-steroidal treatment for uveitis that can decrease reliance on steroids. By using a different approach to calm inflammation, KIO-104 may better preserve vision in uveitis patients.
Kiora expects to reach several key milestones for KIO-104 over the next 1-2 years. In 2023, the company plans to file a pre-IND meeting request with the FDA to align on requirements for initiating a larger phase 2 trial. Kiora also intends to apply for orphan drug designation for KIO-104 to treat non-infectious uveitis in 2023. This designation could provide certain benefits like tax credits and marketing exclusivity incentives. In 2024, Kiora aims to advance KIO-104 into phase 2 clinical trials to further evaluate its efficacy and safety compared to steroid treatments. The phase 2 trial will enroll more patients across multiple study sites to continue gathering data. If the trials meet their endpoints, Kiora anticipates phase 2 results could potentially support the filing of a New Drug Application for KIO-104 in the 2025-2026 timeframe.
Source: Company reports
KIO-104: Phase 1 Study Design

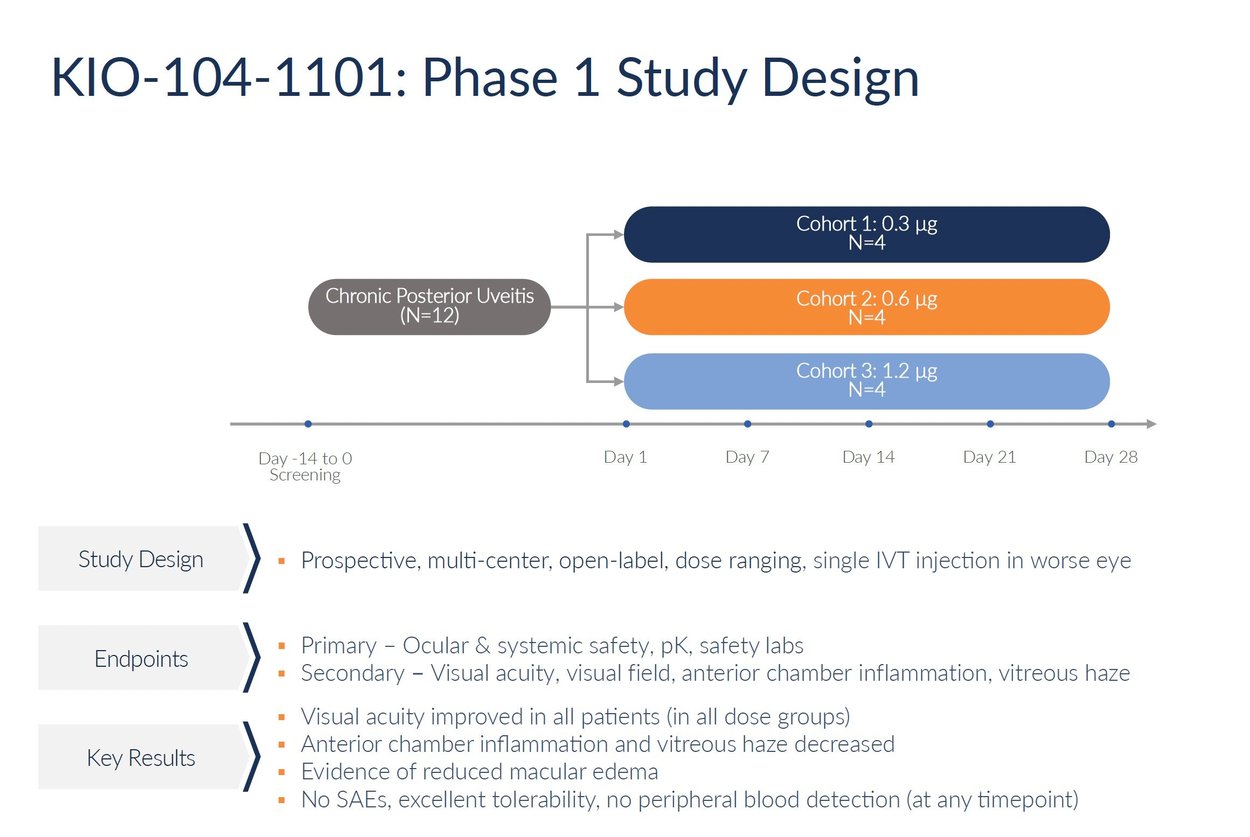
Kiora is preparing to initiate a phase 1 clinical trial evaluating KIO-104 for chronic posterior uveitis. The planned study will be an open-label, multi-center trial assessing the safety and initial activity of a single intravitreal injection of KIO-104. The trial intends to enroll approximately 12 patients across three escalating dose cohorts. Patients will be monitored for four weeks following the injection to evaluate safety outcomes, drug concentrations in the eye, and potential efficacy signals.
The primary objective of the phase 1 study will be to assess the safety and tolerability of KIO-104 delivered by intravitreal injection. Secondary endpoints will look at changes in visual acuity, retinal thickness, vitreous haze grading, and other measures of inflammation and vision changes. The trial aims to determine a target dose range for subsequent testing and provide initial proof-of-concept data. Results will guide the design and endpoints for a larger, placebo-controlled phase 2 trial, pending regulatory clearance.
Source: Company reports
Financings and Capitalization

Financial Position:
Kiora is a clinical-stage pharmaceutical company focused on developing treatments for rare retinal diseases.
As a development-stage biotech company, Kiora continues to invest significantly in research and development as it advances its pipeline. This resulted in a net loss of $10.2 million for the first nine months of 2023, compared to $11.1 million in the first nine months of 2022. The reduced net loss reflects Kiora's commitment to strategically managing expenses while progressing its clinical programs.
As of September 30, 2023, Kiora had $5.4 million in cash and cash equivalents and $1.3 million in tax receivables. Additionally, the company has access to a $10 million equity line of credit, of which approximately $9.6 million remains available.
With multiple clinical trials underway and an experienced leadership team, Kiora maintains a strong financial position to support future growth.
Capitalization:
Kiora continues to have one class of common stock outstanding. As of November 6, 2023, there were 7,689,240 common shares outstanding. Kiora also has various series of preferred stock outstanding that are convertible into additional common shares.
In 2023, Kiora raised $6.3 million through an offering of common stock, preferred stock, and warrants. The company plans to use these proceeds to advance its clinical programs focused on orphan retinal diseases. With strategic capital management, Kiora is positioned to achieve important R&D milestones.
Robust IP and Patent Protection

Kiora's Intellectual Property (IP) portfolio is robust, especially on its pipeline assets, including lead program KIO-301. Patent protection coverage for KIO-301 is projected until 2041.
Patent Portfolio:
-
Kiora has built an intellectual property portfolio around its key product candidates, KIO-101, KIO-201, and KIO-301.
-
The portfolio includes composition-of-matter patents, methods of use patents, and formulations patents.
-
In total, Kiora currently has 26 active and valid patents. The patents are projected to expire between 2023 and 2041.
-
Kiora also has 57 patent applications pending that could provide additional protection if issued.
-
Kiora will continue to file new patent applications to protect innovations and expand its intellectual property.
-
The patents and pending applications help protect Kiora's proprietary product candidates from competition. They give Kiora exclusive rights to develop and commercialize their products.
-
Overall, Kiora is still in the relatively early stages of patent protection.
Pipeline and Upcoming Milestones

With a sharpened strategic focus on developing novel therapies for rare retinal diseases with high unmet needs, Kiora is concentrated on advancing its lead product candidate KIO-301 through clinical trials. As an emerging player targeting overlooked orphan ophthalmic indications, the company has prioritized resources to achieve key milestones for KIO-301 across multiple inherited retinal diseases. In addition, Kiora is progressing KIO-104, a small molecule inhibitor of DHODH, to treat non-infectious uveitis, another rare inflammatory eye disease.
By demonstrating KIO-301's ability to restore lost vision, Kiora aims to transform treatment paradigms for patient populations left behind by current therapies. The company's near-term milestones are focused on generating clinical data and engaging regulators to elucidate the efficacy and safety profiles of both KIO-301 and KIO-104.
Over the next 1-2 years, Kiora expects to reach value-driving milestones that can significantly de-risk these distinct assets while expanding their potential clinical utility. With a patient-focused mission and commitment to efficient orphan drug development, Kiora has outlined a clear clinical strategy intended to capitalize on the differentiated mechanisms of KIO-301 and KIO-104.
KIO-301
The most anticipated milestone is topline data from the Phase 1b ABACUS trial in retinitis pigmentosa patients, now expected in Q4 2023. Positive results showing improved vision would support advancing KIO-301 into Phase 2 trials for RP as well as expanding into choroideremia and Stargardt's disease.
Kiora plans to initiate Phase 2 studies evaluating KIO-301 in RP, choroideremia, and Stargardt's in 2023-2024. These studies will assess the efficacy and optimal dosing. Positive results could support advancing into pivotal Phase 3 trials.
KIO-104
For KIO-104, Kiora is prioritizing development for non-infectious uveitis. The company plans to request a pre-IND meeting with the FDA in 2023 to align on the Phase 2 trial design. Initiation of a Phase 2 trial is expected in 2024.
Strategic Partnerships
Kiora will seek strategic partnerships for its other assets, KIO-101 for ocular inflammation and KIO-201 for corneal defects. This will allow the company to focus resources on orphan retinal programs while benefiting from partners' capabilities to develop the anterior segment programs.
In summary, Kiora has sharpened its pipeline focus on orphan retinal indications led by KIO-301. The company is targeting important data readouts and regulatory milestones over the next 1-2 years that can drive significant value inflection if successful.
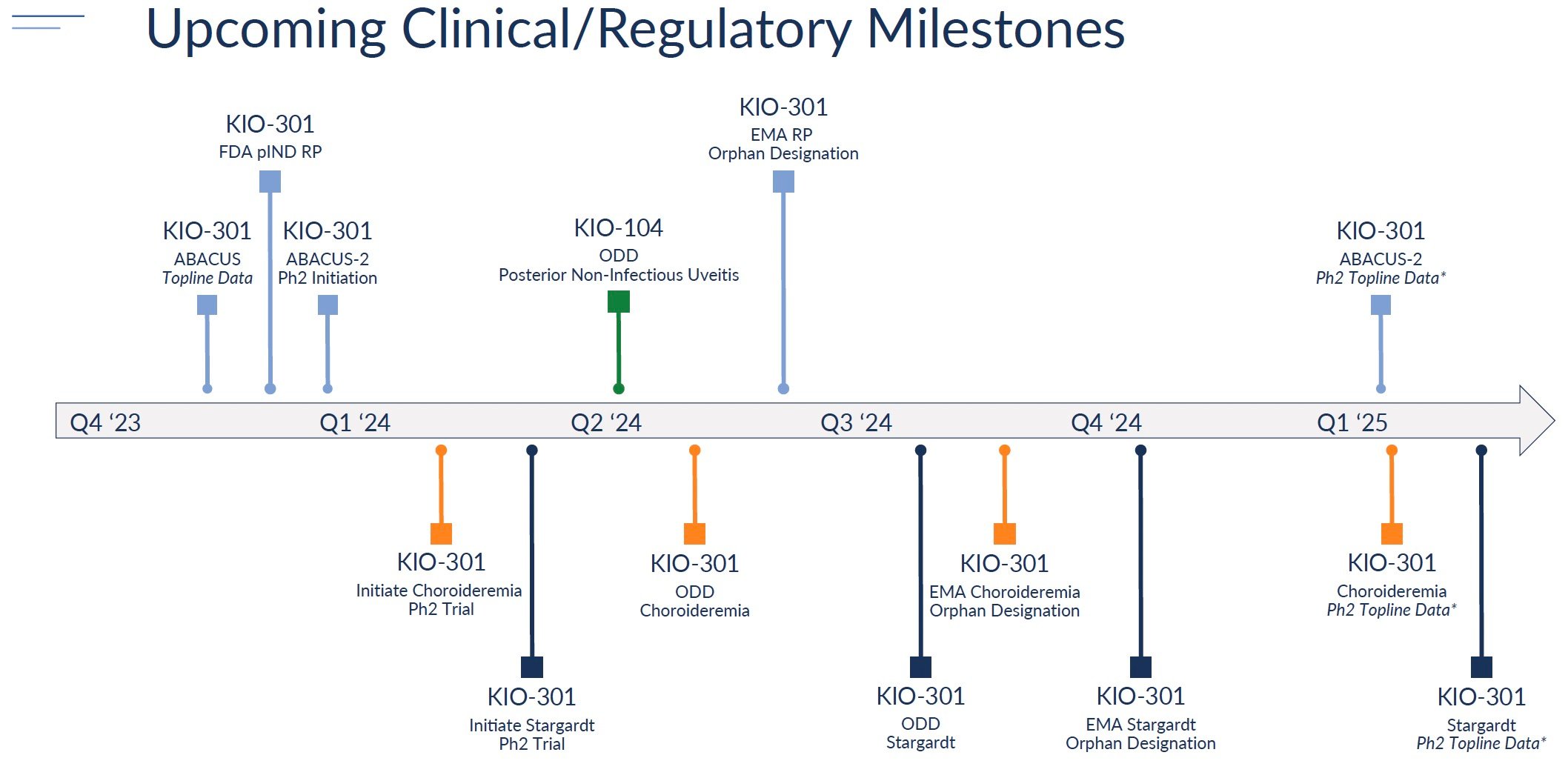 Source: Company reports
Source: Company reports
An Experienced and Proven Management Team

Kiora is led by an experienced and proven management team:
Brian M. Strem, PhD, President and CEO
Dr. Strem was a co-founder and Director of Bayon Therapeutics, Inc., and Okogen, Inc. Prior to founding Okogen, Brian worked at Sound Pharmaceuticals, Allergan, and Shire, where he was responsible for business development and corporate strategy in ophthalmology, ontology, and regenerative medicine. Brian began his career at Cytori Therapeutics with elevating roles within the commercial and R&D departments. Brian received his BS in Bioengineering from Cornell University and his Ph.D. in Biomedical Engineering from UCLA.
Eric J. Daniels, MD, MBA, Chief Development Officer
Dr. Daniels has nearly 20 years of operating experience in biotechnology and medtech companies. Prior to joining Kiora, he served as the CEO of OccuRx Pty Ltd as well as Co-founder and Director of Bayon Therapeutics, Inc., both focused on the development of next-generation ophthalmic therapeutics. Eric is also a Co-Founder and Director of Okogen, Inc., a specialty ophthalmic biotechnology company. Eric previously served as part of the leadership teams at Puregraft LLC, Tensys Medical, and Cytori Therapeutics, Inc., serving in senior medical, R&D, and commercial roles. Eric holds an MBA from the Anderson School of Management at UCLA, where he also received his MD from the UCLA School of Medicine. Eric received his BS in Molecular Biology from the University of California, Berkeley.
Melissa Tosca, CPA, EVP of Finance
Ms. Tosca Melissa Tosca is the Executive Vice President of Finance for Kiora Pharmaceuticals. She has 22 years of public accounting and finance experience, including 15 years at biotechnology and life science companies. Prior to joining Kiora, Melissa served as Executive Director of Finance and Corporate Treasurer for Neomorph, where she managed the company’s finance and accounting functions. Prior to Neomorph, Melissa served as Director of Finance and Accounting at Omniome, where she built the accounting and finance infrastructure and managed the company’s financial operations. Prior to Omniome, she spent 9 years at Caris Life Sciences, serving in various leadership roles, including Director of Finance and Accounting, Director of Financial Planning and Analysis, and Senior Director of Sales Operations. Melissa began her professional career in public accounting at Clifton Gunderson and later at Ernst and Young as an Audit Manager. Melissa is a Certified Public Accountant and holds a B.S. in Accounting from the University of Arizona.
Stefan Sperl, PhD, EVP of CMC and Operations
Dr. Sperl acts as Executive Vice President of CMC and Operations at Kiora. Previously, he was the co-founder and COO of Panoptes Pharma. Prior to this position, he was the global Project Manager at Nabriva Therapeutics AG, responsible for the development of XENLETATM from early preclinical to clinical phase 2 programs. Before that, he had various roles in R&D at Biovertis AG, Austria, and Wilex AG, Germany. He has profound knowledge of drug development and is an inventor with numerous patents. Dr. Stefan Sperl received his PhD in Medicinal Chemistry from the Max Planck Institute in Martinsried, Germany.
Source: Company Reports
Strategically Located in San Diego Biotech Hub

San Diego has become one of the largest biotechnology hubs in the world, second only to the Boston/Cambridge area. Over 900 life sciences companies, including large pharmaceutical companies, emerging startups, research institutes, and support services, are based in San Diego.
Major players located in San Diego include Illumina, Thermo Fisher Scientific, BD Biosciences, IQVIA, and Vertex Pharmaceuticals. Dozens of clinical-stage biotechs are also headquartered there.
The concentration of biopharma in San Diego is no accident. The area boasts prestigious research centers, including UC San Diego, Scripps Research, Salk Institute, and Sanford Burnham Prebys Medical Discovery Institute. This dense talent pipeline entices companies.
San Diego also offers a high quality of life, drawing top scientists and executives. The ecosystem creates fruitful collaboration opportunities for startups through local networking.
For an emerging biotech like Kiora Pharmaceuticals, being based in San Diego provides valuable proximity to potential partners, academic collaborators, industry experts, and investors focused on life sciences.
The cluster effect of having so many interconnected companies accelerates innovation, recruitment, and funding opportunities. San Diego's biotech culture also promotes the entrepreneurial drive and boundary-pushing vision that leads to breakthroughs.
Overall, Kiora's headquarters location gives it key strategic advantages, access to specialized talent and resources, and positioning among the vibrant heart of cutting-edge bioscience advancement.
SEC Filings
Financials
Risks & Disclosures

This communication is neither an offer to sell nor a solicitation of an offer to buy, nor a recommendation of any securities of the company mentioned herein.
Kiora Pharmaceuticals, Inc. (the “Company”) has reviewed the content of this page as well as the accompanying presentation (“Company Presentation”) displayed on this page. To the best of its knowledge, the Company does not believe this content to be misleading or inaccurate in any material respect, nor does it believe there are any material omissions with respect to such content. The Company does not believe the contents of the page or the Company Presentation to contain any non-public material information.
Information and opinions presented in the Company Presentation are provided by the Company, and b2iDigital makes no representation as to their accuracy or completeness. The information contained on this page is not intended to constitute any form of advice, and the information provided is not intended to provide a sufficient basis on which to make an investment decision. It is not investment research, nor does it constitute a research recommendation, as it does not constitute substantive research or analysis. This information is not to be relied upon in substitution for the exercise of independent judgment.
Information, opinions and estimates contained on this page or in the Company Presentation reflect judgments by the Company as of the original date of publication by the Company and are subject to change without notice. Past performance should not be taken as an indication or guarantee of future performance, and no representation or warranty, express or implied is made regarding future performance.
A complete description of the risks and uncertainties relating to the Company and its securities can be found in the company's filings with the U.S. Securities and Exchange Commission available for free at www.sec.gov.
Information on this page may relate to penny stocks, which may also be referred to as low-priced stocks. Penny stocks are low-priced shares typically issued by small companies. Penny stocks involve greater than normal risk, they may be less liquid than other stocks (i.e., more difficult to sell), and there may be less reliable information available regarding such stocks. Investors in penny stocks should be prepared for the possibility that they may lose their entire investment.
b2i digital or its related entities may own securities of the Company.
The Company is a client of b2i Digital. The Company agreed to pay b2i Digital no greater than $100,000 in cash for 12 months of digital marketing consulting and investor awareness services.
This communication includes forward-looking statements that involve risks, uncertainties and assumptions that are difficult to predict. Words and expressions reflecting optimism, satisfaction or disappointment with current prospects, as well as words such as “believes,” “hopes,” “intends,” “estimates,” “expects,” “projects,” “plans,” “anticipates” and variations thereof, or the use of future tense, identify forward-looking statements, but their absence does not mean that a statement is not forward-looking. The Company’s forward-looking statements are not guarantees of performance, and actual results could vary materially from those contained in or expressed by such statements due to risks, uncertainties and other factors. The Company urges readers to consider specifically the various risk factors identified in its most recent Form 10-K, and any risk factors or cautionary statements included in any subsequent Form 10-Q or Form 8-K, filed with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this communion. Except as required by law, the Company does not undertake any responsibility to update any forward-looking statements to take into account events or circumstances that occur after the date of this communication.
The Kiora Pharmaceuticals management and investor relations team are available to talk to current and potential investors. They're happy to answer your questions and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
• Directly hear the Kiora Pharmaceuticals story
• Ask your questions
• Submit the form below, and someone will get in touch with you as soon as possible
Note: Company management or its representative can only discuss and disclose information that is already available in the public domain. They will do their best to clarify such information to the extent permitted by securities law and industry regulations.














Restoring vision in blindness diseases like Retinitis Pigmentosa (RP) with KIO-301
KIO-301 is a potential vision-restoring medicine for blindness diseases like RP. It is designed to transform downstream cells in the retina to become light-sensitive. RP causes loss of photoreceptors, but KIO-301 enters these living downstream retinal cells and makes them able to signal the brain to restore vision. Early clinical results show improved light detection in RP patients after treatment.