AIM ImmunoTech, Inc.
AIM ImmunoTech Inc. is an immuno-pharma company primarily engaged in developing therapeutics for various significant diseases, including multiple types of cancers, immune disorders, and viral infections like COVID-19. The company's flagship product, Ampligen® (rintatolimod), is an immune-modulator under development for various indications. It's notable for its broad-spectrum activity and is currently involved in numerous clinical studies aimed at demonstrating its efficacy across multiple high-value disease areas, including oncology and chronic viral diseases.
NYSEAMERICAN: AIM
IR Website: aimimmuno.com
Headquarters: Ocala, FL
Investor Contact: aim@jtcir.com
TALK TO MANAGEMENT
AIM ImmunoTech Inc. is always available to talk to current and potential investors. They're happy to answer any questions you may have and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
Summary

AIM ImmunoTech Inc., an immuno-pharma company based in Ocala, Florida, is dedicated to the development of therapeutics for various cancers, immune deficiency disorders, and viral diseases, including COVID-19. The company has built a substantial portfolio of laboratory, pre-clinical, and clinical data focusing on nucleic acids and natural interferon to boost the human body's antiviral defenses and assist in developing therapies for specific cancers and chronic conditions.
AIM ImmunoTech's leading products are Ampligen® (rintatolimod) and Alferon N Injection® (Interferon alfa-n3). Ampligen® is a pioneering RNA-based drug targeting significant global health challenges, including various cancers and viral diseases. It is approved in Argentina for severe chronic fatigue syndrome (CFS) and is under evaluation for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and post-COVID conditions. Alferon N Injection® is approved in the U.S. for the intralesional treatment of refractory or recurrent external genital warts in adults and for a specific STD infection in Argentina, noted for being the only natural-source, multi-species alpha interferon approved for these conditions.
Press Releases
Social Media Updates

Recent LinkedIn Posts
Recent Facebook, Instagram, Pinterest, TikTok, and X Posts
Investor Presentation


To download the AIM ImmunoTech Inc. investor presentation, please fill out the form below.
Stock Chart (Intraday)
Stock Chart (Historical)
CEO Corner Videos & Virtual Investor Series

The CEO Corner platform was launched in March 2024 to provide an additional and in-depth perspective on press releases, corporate developments, and development pipeline progress.
“Keeping our stakeholders well informed is absolutely essential to the future success of AIM ImmunoTech. CEO Corner is a new way for us to connect directly with both current and potential stockholders. CEO Corner segments will provide expanded perspective on press releases, clinical trials and other corporate advancements as we continue to push forward with our development pipeline. We believe in the future of Ampligen and are dedicated to growing value for all stakeholders," said CEO Tom Equels.
March 7, 2024 –
Welcome to the AIM CEO Corner
March 13, 2024 –
Oncology Overview
March 20, 2024 –
Ovarian Cancer
March 20, 2024 –
Virtual Investor Lunch Break: The AIM Opportunity
April 11, 2024 –
Top-line Interim Data from Recurrent Ovarian Cancer Study
April 25, 2024 –
Metastatic Pancreatic Cancer
July 3, 2024 –
Corporate and Clinical Update
July 29, 2024 –
Early Stage Triple Negative Breast Cancer
Videos Source: Company Reports
Ampligen® - A Wide Variety of Potential Applications

AIM ImmunoTech has strategically positioned itself as a leader in immunotherapy research and development. With a focus on leveraging the unique properties of its flagship drug candidate, Ampligen, the company is addressing critical unmet needs across multiple therapeutic areas. This comprehensive approach not only diversifies AIM's potential market impact but also maximizes the opportunities for breakthrough treatments in complex diseases.

Source: Company Reports
Ampligen, the lead investigational drug developed by AIM ImmunoTech, is currently being evaluated across various indications in multiple human clinical trials. The drug is under investigation for potential efficacy in several oncology applications, including locally advanced and metastatic pancreatic cancer, as well as advanced recurrent ovarian cancer, both as a standalone treatment and in combination with other therapies. Notably, Ampligen is being studied in combination with established immunotherapies like Durvalumab and Pembrolizumab. The company has also reported positive data from an Early Access Program for late-stage pancreatic cancer in the Netherlands and is investigating Ampligen's potential in treating Long COVID / Post-COVID Conditions. Across these trials, AIM ImmunoTech is actively enrolling and dosing patients, reporting interim results, and progressing through various clinical phases, demonstrating a robust and diverse clinical development program for Ampligen.
AIM ImmunoTech's lead investigational drug, Ampligen® (rintatolimod), stands out as a novel immunotherapeutic agent with a distinctive mechanism of action. As noted below, Ampligen is being investigated for its potential antitumor applications.
 Source: Company Reports
Source: Company Reports
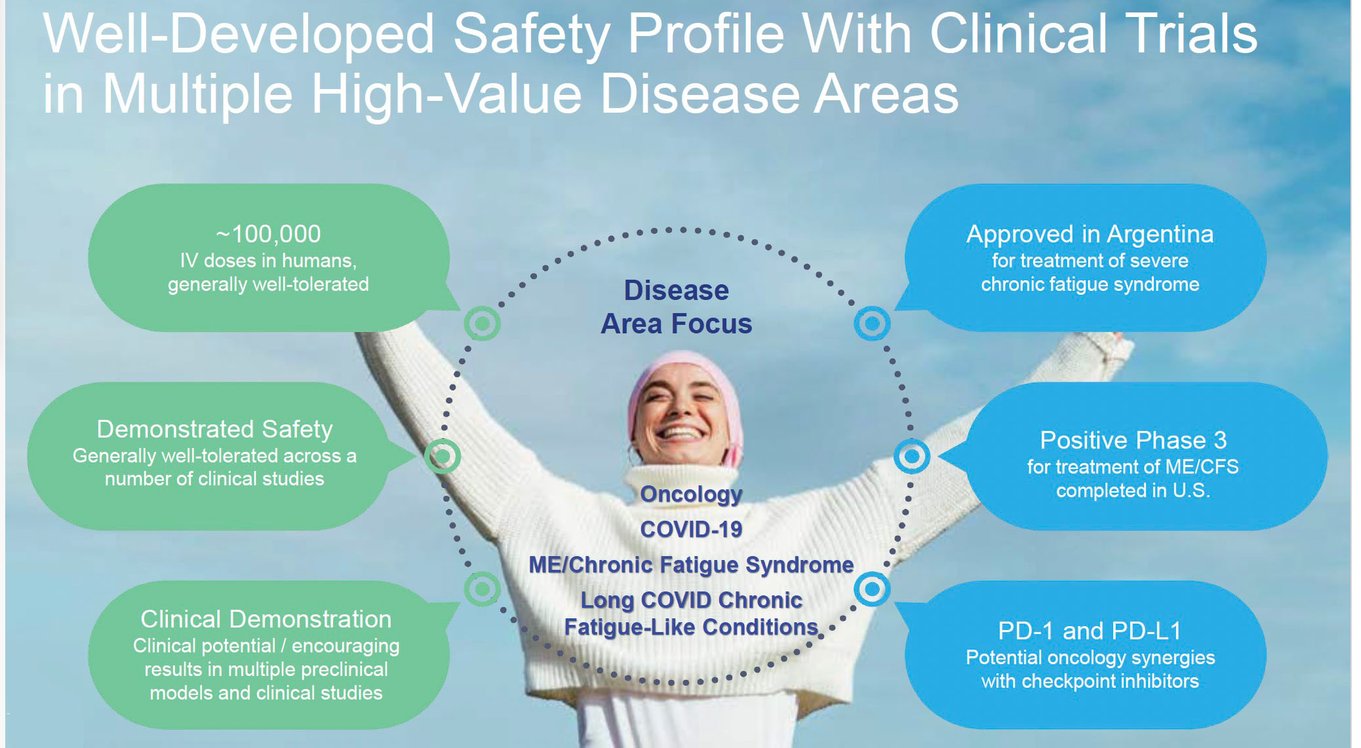
Ampligen's safety profile is being continuously evaluated across multiple disease areas. With approximately 100,000 IV doses administered in humans, the drug has demonstrated general tolerability and encouraging results in various preclinical models and clinical studies. Ampligen's potential applications span oncology, COVID-19, ME/Chronic Fatigue Syndrome, and Long COVID conditions, showcasing its versatility as a therapeutic candidate. Ampligen is approved in Argentina for severe chronic fatigue syndrome, and a Phase 3 trial for ME/CFS in the United States has been completed with ongoing analysis.
Source: Company Reports
Next Steps:
AIM ImmunoTech reports ongoing positive developments in its cancer treatment programs with Ampligen. Recent studies continue to explore the potential for enhanced anti-tumor immunity in recurrent ovarian cancer. Recent findings related to Ampligen's study in pancreatic cancer have been reported and are under review for publication. The company continues to explore and expand Ampligen’s therapeutic potential across various oncological applications.
In the video below, CEO Thomas Equels joined Proactive's Stephen Gunnion after the company announced the start of an NCI-funded Phase 2 study of its product candidate, Ampligen, in melanoma. Equels gave an overview of Ampligen and explained the process for the Phase 2 study and the steps AIM will take if it is successful. He also provided an overview of AIM's other programs with Ampligen.
Ampligen® - Mechanism in Oncology

Ampligen's unique mechanism of action in oncology focuses on reprogramming the tumor microenvironment to enhance the immune response against cancer cells. By converting "cold" tumors into "hot" tumors, Ampligen aims to make cancers more responsive to checkpoint blockade therapies. This approach leverages Ampligen's ability to selectively attract cancer-fighting T cells while reducing regulatory T cells that can suppress immune responses, potentially offering a novel strategy to improve cancer treatment outcomes.

Source: Company Reports
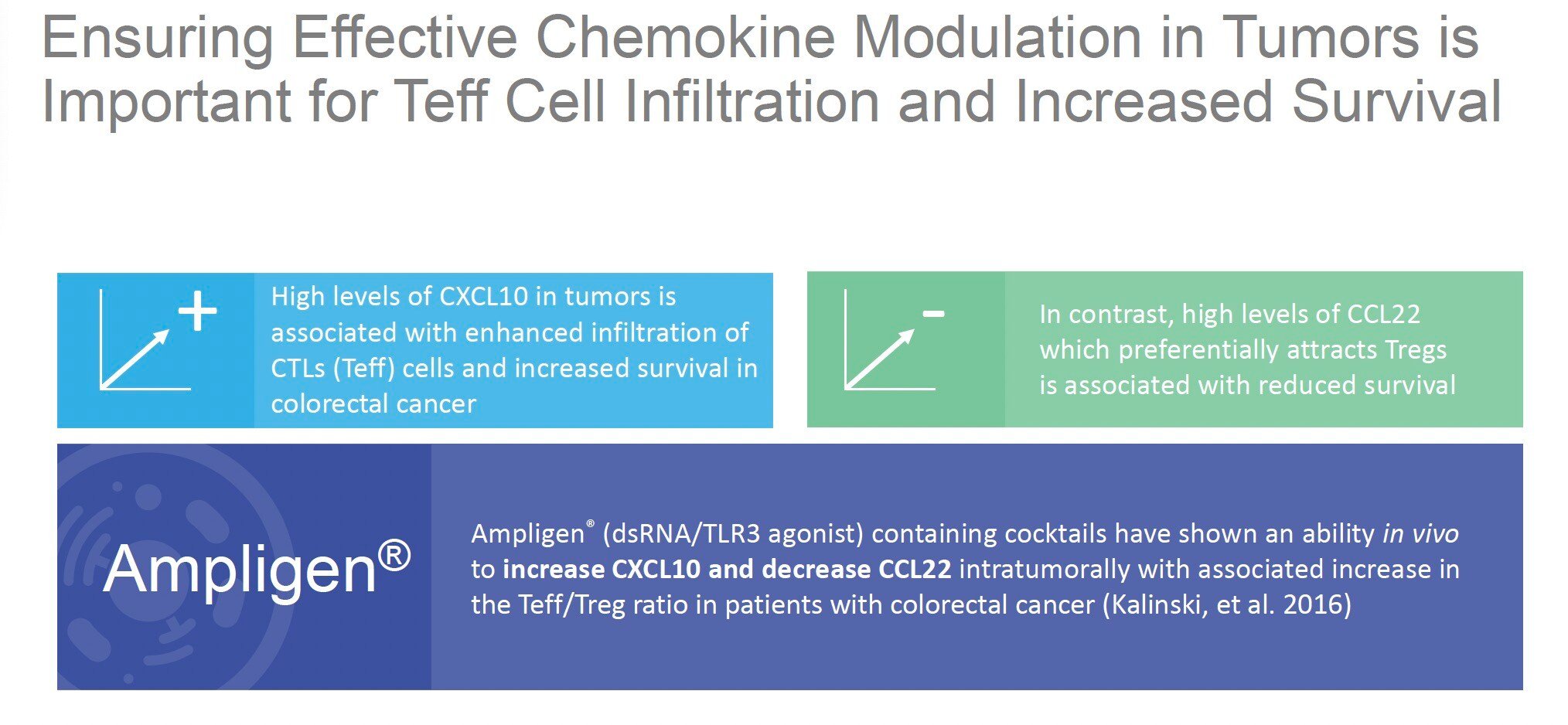
Chemokine modulation plays a crucial role in shaping the tumor microenvironment and influencing cancer outcomes. By altering the balance of specific chemokines, such as increasing CXCL10 and decreasing CCL22, therapies like Ampligen can potentially enhance the infiltration of cancer-fighting T cells (Teff) while reducing immunosuppressive regulatory T cells (Tregs). This modulation aims to create a more favorable immune environment within tumors, potentially leading to improved patient survival rates.
Source: Company Reports
Ampligen demonstrates a consistent pattern of immune activation across various solid tumor types, suggesting a broad applicability in oncology. This table illustrates how Ampligen influences key markers associated with T-cell chemotaxis and infiltration in different cancers, including ovarian, colorectal, triple-negative breast, and pancreatic cancers. The similarity in responses across these diverse tumor types underscores Ampligen's potential as a versatile immunotherapy agent.

Source: Company Reports
Ampligen® - In Oncology Programs

Ampligen has shown promising results in multiple cancer types, with a particular focus on pancreatic cancer. AIM ImmunoTech is conducting several clinical trials to evaluate Ampligen's efficacy in various stages of pancreatic cancer, as well as in other solid tumors such as ovarian, breast, colorectal, and melanoma.

Source: Company Reports
Pancreatic cancer represents a significant unmet medical need, with approximately 62,000 new cases and 50,000 deaths annually in the United States. The high mortality rate underscores the urgent need for new, effective treatments in this area.

Source: Company Reports
AIM ImmunoTech is pursuing two Phase 2 clinical trials in pancreatic cancer. AMP-270 is evaluating Ampligen following FOLFIRINOX in locally advanced pancreatic adenocarcinoma, while DURIPANC is studying the combination of Ampligen and durvalumab in metastatic pancreatic ductal adenocarcinoma.

Source: Company Reports
AIM ImmunoTech has established strategic collaborations to advance Ampligen's development in oncology. These partnerships leverage the expertise of leading institutions and companies, enhancing the potential for successful outcomes in clinical trials across various cancer types, including advanced and recurrent ovarian cancer.

Source: Company Reports
Ampligen® - In Pancreatic Cancer Programs

Ampligen has shown promising results in treating pancreatic cancer, one of the most challenging malignancies. AIM ImmunoTech is pursuing multiple clinical pathways to evaluate Ampligen's efficacy in this critical area of unmet medical need. Promising results from an Early Access Program at Erasmus University showed a statistically significant increase in overall survival for late-stage pancreatic cancer patients treated with Ampligen compared to historical controls.
The Early Access Program for Ampligen in late-stage pancreatic cancer, conducted in partnership with Erasmus MC, has yielded encouraging results. With over 55 subjects enrolled, this ongoing study uses Ampligen as monotherapy. Positive results published in March 2022 indicate a significant improvement in progression-free survival compared to historical controls.
Early Access Program in Late-Stage Pancreatic Cancer - Overall Survival] Further analysis of the Early Access Program data demonstrates promising overall survival outcomes. The graphs show improved survival probabilities for patients treated with Ampligen compared to historical controls, both in the general patient population and in subgroups such as those with CA19.9 levels below 1000. These results suggest Ampligen's potential to extend life in late-stage pancreatic cancer patients.
Source: Company Reports
Ampligen® - As a Potential Treatment for Post-COVID Conditions: Fatigue

AIM ImmunoTech's research into Ampligen as a potential treatment for post-COVID conditions focuses on two key areas: fatigue and cognitive dysfunction. The results are summarized below in four slides.
The Phase 2 study (AMP-518) demonstrated promising results in reducing fatigue, with Ampligen outperforming placebo in PROMIS® fatigue measures and the Six-Minute Walk Test. Preliminary data from the Hunter-Hopkins center showed improvements in exercise ability, fatigue levels, and post-exertional malaise over 12 weeks. Additionally, early results indicate potential benefits in cognitive function, with patients reporting improvements in concentration, memory, and overall cognitive performance after 24 weeks of treatment. These findings suggest that Ampligen may offer a comprehensive approach to addressing the complex symptoms of long-COVID, potentially improving both physical and cognitive outcomes for patients.
AIM ImmunoTech conducted a Phase 2 study to evaluate Ampligen's effectiveness in treating post-COVID fatigue. 80 subjects were enrolled in the study, and the preliminary topline data were reported in February 2024.
Source: Company Reports
Preliminary data from the Hunter-Hopkins Center provide encouraging results for Ampligen in treating long-term COVID-19 symptoms. As shown below, patient-reported outcomes demonstrate improvements in exercise ability, fatigue levels, and post-exertional malaise over a 12-week period.
 Source: Company Reports
Source: Company Reports
The topline results from the AMP-518 study suggest Ampligen's potential in alleviating post-COVID fatigue. The slide below summarizes key findings, including Ampligen's performance against placebo in PROMIS® fatigue measures and the Six-Minute Walk Test, as well as its safety profile.

Source: Company Reports
Below are preliminary data from the Hunter-Hopkins center on Ampligen's potential to improve cognitive dysfunction in long-COVID patients. The results show improvements in measures of cognitive function, including difficulty concentrating or focusing, memory problems, and impaired memory or concentration. The data compares baseline scores to those after 24 weeks of treatment, suggesting Ampligen may help alleviate these persistent cognitive symptoms associated with long-COVID.

Source: Company Reports
Market Potential

AIM ImmunoTech believes that it has potential solutions for a number of different indications, both for use in isolation as well as in conjunction with another drug.
First, Ampligen is in late-stage trials for the treatment of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Up to 2.5 million Americans are estimated to suffer from this malady, causing the U.S. economy an estimated $17 to $24 billion per year, according to the CDC.
Additionally, Ampligen is currently being studied for alleviating "long COVID"—the long-term issues some COVID patients suffer from. While there is not much long-term information to draw from, a recent UCLA study implies that around 30% of COVID patients have long-term symptoms.
Lastly, oncology: AIM ImmunoTech believes that it may have therapeutic solutions for numerous types of cancer. As a malady that impacts so many of us, the potential market for these therapeutics is enormous. Precedence Research estimates that the global market will balloon to over $580 billion annually in 2030, with a Compounded Annual Growth Rate (CAGR) of 8.2% this decade.
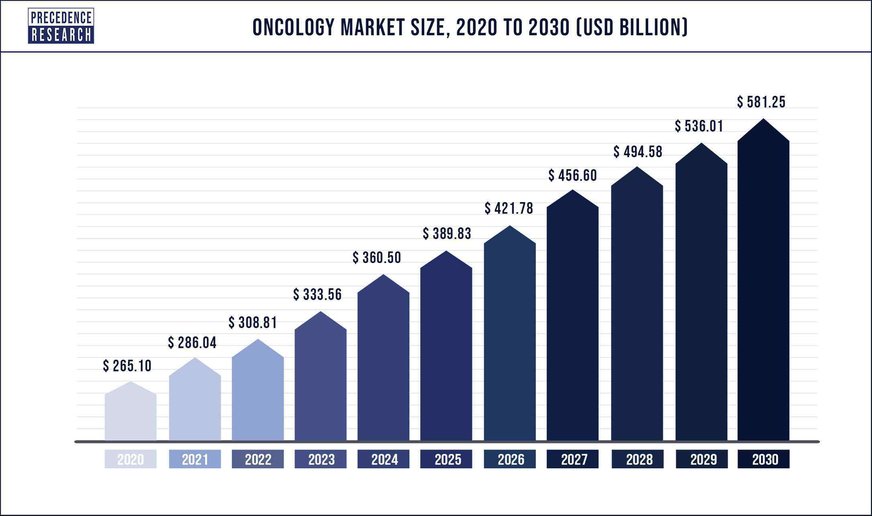 Source: Precedence Research
Source: Precedence Research
Ampligen® Current Pipeline

Ampligen, the lead investigational drug developed by AIM ImmunoTech, is currently being evaluated across various indications in multiple human clinical trials. The drug is under investigation for potential efficacy in several oncology applications, including locally advanced and metastatic pancreatic cancer, as well as advanced recurrent ovarian cancer, both as a standalone treatment and in combination with other therapies. Notably, Ampligen is being studied in combination with established immunotherapies like Durvalumab and Pembrolizumab. The company has also reported positive data from an Early Access Program for late-stage pancreatic cancer in the Netherlands and is investigating Ampligen's potential in treating Long COVID / Post-COVID Conditions. Across these trials, AIM ImmunoTech is actively enrolling and dosing patients, reporting interim results, and progressing through various clinical phases, demonstrating a robust and diverse clinical development program for Ampligen.

Source: Company Reports
Alferon N Injection®

Alferon N Injection® continues to be an FDA-approved treatment for refractory or recurrent external condylomata acuminata. The commercial relaunch is pending the completion of the FDA's manufacturing approval process, including a successful pre-approval inspection. Sales are expected to resume following a successful pre-approval inspection and obtaining the necessary supplemental approvals.
Strong Cash Position & Poised for Commercialization

For biotech companies, securing sufficient funding is crucial to sustain development and regulatory approval processes, which are both time-intensive and costly. A lack of adequate funding is a common hurdle that prevents many biotech firms from bringing their candidates to market.
As of Q2 2024, AIM ImmunoTech is in a favorable position compared to many of its peers. With cash and cash equivalents of more than $10.0 million, the company remains strategically poised with enough financial resources to pursue its clinical development goals and move towards the commercialization of its products.
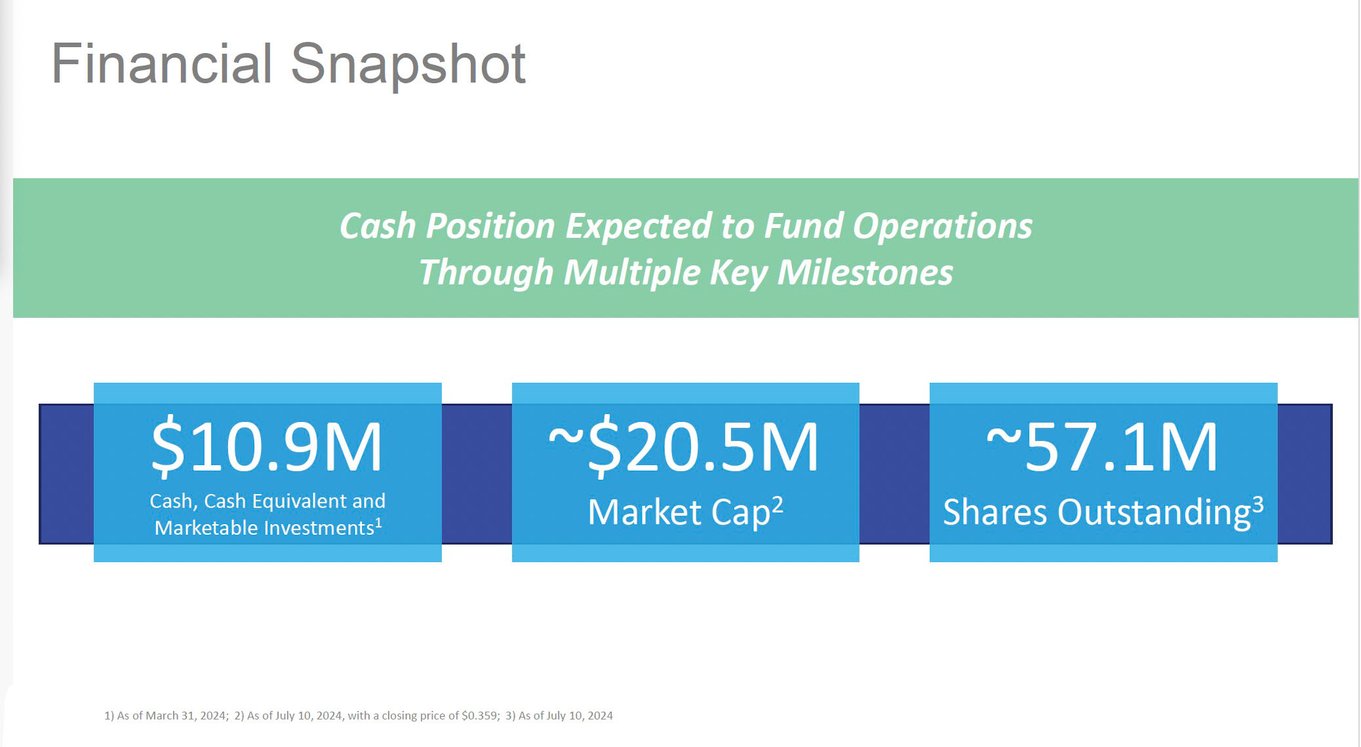
Source: Company Documents
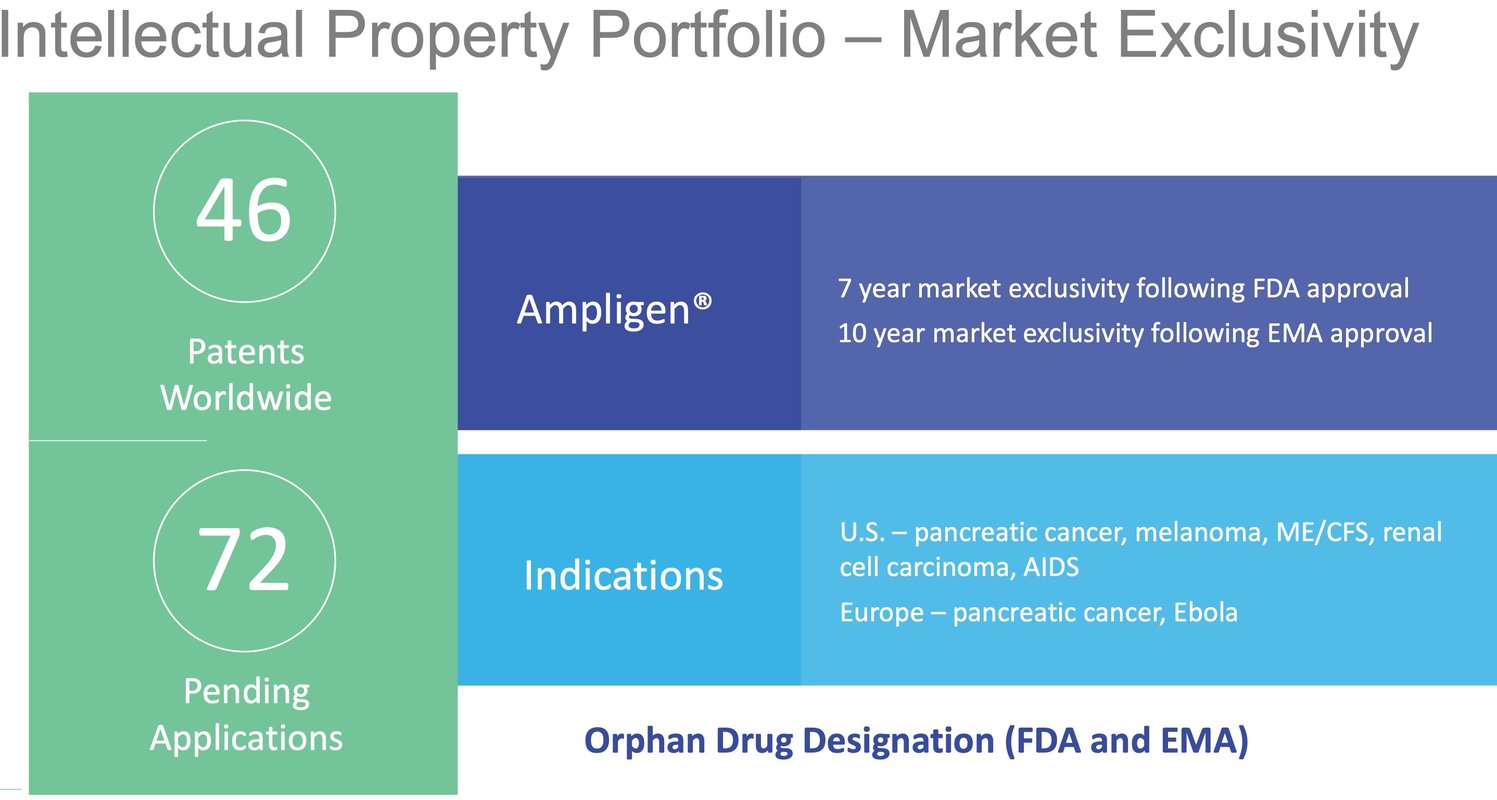
While a strong cash position is no guarantee that its candidates will successfully move through the regulatory approval process, its cash position combined with a strong intellectual property stack puts it in a strong position for future success.
AIM ImmunoTech’s intellectual property portfolio is robust, with 46 patents worldwide, providing strong protection for its drug candidates. Ampligen, the company’s flagship product, benefits from market exclusivity, offering seven years of protection following FDA approval in the U.S. and ten years following EMA approval in Europe. This strategic combination of patents and market exclusivity positions AIM ImmunoTech to capitalize on its innovations, ensuring a competitive edge in the biotech market.
Source: Company Reports
SEC Filings
Risks & Disclosures

This communication is neither an offer to sell nor a solicitation of an offer to buy, nor a recommendation of any securities of the company mentioned herein.
AIM ImmunoTech Inc. (the “Company”) and its counsel have reviewed the content of this page as well as the accompanying presentation (“Company Presentation”) displayed on this page. To the best of its knowledge, the Company does not believe this content to be misleading or inaccurate in any material respect, nor does it believe there are any material omissions with respect to such content. The Company does not believe the contents of the page or the Company Presentation to contain any non-public material information.
Information and opinions presented in the Company Presentation are provided by the Company, and makes no representation as to their accuracy or completeness. The information contained on this page is not intended to constitute any form of advice, and the information provided is not intended to provide a sufficient basis on which to make an investment decision. It is not investment research, nor does it constitute a research recommendation, as it does not constitute substantive research or analysis. This information is not to be relied upon in substitution for the exercise of independent judgment.
Information, opinions and estimates contained on this page or in the Company Presentation reflect judgments by the Company as of the original date of publication by the Company and are subject to change without notice. Past performance should not be taken as an indication or guarantee of future performance, and no representation or warranty, express or implied is made regarding future performance.
A complete description of the risks and uncertainties relating to the Company and its securities can be found in the company's filings with the U.S. Securities and Exchange Commission available for free at www.sec.gov.
Information on this page may relate to penny stocks, which may also be referred to as low-priced stocks. Penny stocks are low-priced shares typically issued by small companies. Penny stocks involve greater than normal risk, they may be less liquid than other stocks (i.e., more difficult to sell), and there may be less reliable information available regarding such stocks. Investors in penny stocks should be prepared for the possibility that they may lose their entire investment.
B2i Digital or its related entities may own securities of the Company. Specifically, the CEO of B2i Digital owns 10,000 shares of AIM ImmunoTech, Inc. as of August 18, 2024.
The Company is a client of B2i Digital. The Company agreed to pay B2i Digital no greater than $100,000 in cash for 12 months of digital marketing consulting and investor awareness services.
This communication includes forward-looking statements that involve risks, uncertainties and assumptions that are difficult to predict. Words and expressions reflecting optimism, satisfaction or disappointment with current prospects, as well as words such as “believes,” “hopes,” “intends,” “estimates,” “expects,” “projects,” “plans,” “anticipates” and variations thereof, or the use of future tense, identify forward-looking statements, but their absence does not mean that a statement is not forward-looking. The Company’s forward-looking statements are not guarantees of performance, and actual results could vary materially from those contained in or expressed by such statements due to risks, uncertainties and other factors. The Company urges readers to consider specifically the various risk factors identified in its most recent Form 10-K, and any risk factors or cautionary statements included in any subsequent Form 10-Q or Form 8-K, filed with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this communion. Except as required by law, the Company does not undertake any responsibility to update any forward-looking statements to take into account events or circumstances that occur after the date of this communication.
Management Team

Thomas K Equels, M.S., J.D.
Thomas K Equels, M.S., J.D.
Chief Executive Officer, President & Executive Vice Chairman
THOMAS K. EQUELS, M.S., J.D. has been the Chief Executive Officer of AIM ImmunoTech Inc. since February 2016. Additionally, he has served as a Director on the company’s Board of Directors since 2008. Mr. Equels previously served as the company’s Secretary from 2008 to 2016, its General Counsel from 2010 to 2016, and its Chief Financial Officer from December 2013 to February 2016. Mr. Equels was formerly the President and Managing Director of the Equels Law Firm in Miami, Fla. For over a quarter century, he represented national governments, state governments and private companies in banking, insurance, aviation, pharmaceutical and construction matters. He also was on numerous occasions the court-appointed receiver to turn around distressed companies. Mr. Equels received his Juris Doctor degree with high honors from Florida State University. He is a summa cum laude graduate with a Bachelor of Science from Troy University. He also has a Master of Science from Troy University. He is a member of the Florida Bar Association and the American Bar Association.

Peter W. Rodino III, J.D.
Peter W. Rodino III, J.D.
Chief Operating Officer, Executive Director for Governmental Relations, General Counsel, Secretary
PETER W. RODINO, III, J.D., was named Executive Director for Governmental Relations and General Counsel in October 2016 and Secretary in November 2016. Mr. Rodino was appointed a Director of the Company in July 2013 and served in that capacity until his resignation to permit him to serve the company in this new capacity. While on the Board, he served as Lead Director, Chairman and Financial Expert of the Audit Committee; as a member of the Compensation Committee; and as a member of the Governance and Nomination Committee. Mr. Rodino has broad legal, financial and executive experience. In addition to being President of Rodino Consulting LLC and managing partner at several law firms during his many years as a practicing attorney, he served as Chairman and CEO of Crossroads Health Plan, the first major Health Maintenance Organization in New Jersey. He also has experience as an investment executive in the securities industry and acted as trustee in numerous Chapter 11 complex corporate reorganizations. Mr. Rodino holds a Juris Doctor degree from Seton Hall University and a Bachelor of Science in Business Administration from Georgetown University.

David R. Strayer, M.D.
David R. Strayer, M.D.
Medical Officer
Dr. Strayer has acted as Medical Director since 1986 and was appointed Medical Officer in 2016. He is a former Professor of Medicine and Neoplastic Diseases at Hahnemann University. Dr. Strayer is Board Certified in Medical Oncology and Internal Medicine with research interests in the fields of cancer and immune system disorders. He has served as principal investigator in studies funded by the Leukemia Society of America, the American Cancer Society, and the National Institutes of Health. Dr. Strayer attended the School of Medicine at the University of California at Los Angeles where he received his M.D. in 1972.

Robert Dickey IV, MBA
Robert Dickey IV, MBA
Chief Financial Officer
ROBERT DICKEY IV has more than 25 years of experience of C-suite financial leadership for life science and medical device companies, both private and public, ranging from preclinical development to commercial operations and across a variety of disease areas and medical technologies.

Christopher McAleer, Ph.D.
Christopher McAleer, Ph.D.
Scientific Officer
Dr. McAleer has acted as Scientific Officer since his appointment in December 2022. Prior to joining the Company in June 2022 as Deputy Scientific Officer, he held positions at Hesperos, Inc., where he helped to build a start-up biotech CRO from a 3-employee $500K/year operation to a 30 person $5+ million/year operation. In his most recent role at Hesperos as Principal Scientist Dr. McAleer was responsible for leading teams in higher throughput operations projects as well as prototype projects including both NIH-funded and industry-sponsored which included studies of neuromuscular disorders, human complement pathways, cardiac dysfunction, liver metabolism and PKPD modeling, M/NAFLD, sarcopenia, and metabolic syndrome, and autoimmune diseases. Prior to his appointment as Principal Scientist, he served as Senior Scientist from 2016-2019 where he led a team to build a multi-organ MPS-based model using human and rat primary and iPSC derived tissues to study interspecies differences of drug toxicity. Additionally, as Senior Scientist he was responsible for writing in full or in part 7 funded NIH SBIR grant proposals as well as executing 4 contracts for private client partners resulting in repeat business from all 4 clients totaling ~$1.3 million. Additionally, Dr. McAleer has authored published manuscripts in several peer-reviewed science and nature journals, book chapters and scientific conference publications. His manuscript titled “Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics,” published in Science Translational Medicine in December 2019 had the distinction of being featured by then-NIH director Francis Collins’ weekly blog.
Dr. McAleer received his Bachelor of Science Education in Biology and Chemistry degrees from West Chester University of Pennsylvania, and his Masters in Molecular Biology and Microbiology as well as his Doctorate in Biomedical Sciences from University of Central Florida. He completed his post-doctoral research at the University of Central Florida.
AIM ImmunoTech, Inc. is led by a team of dedicated professionals with extensive pharmaceutical development expertise.
The AIM ImmunoTech, Inc. team regularly updates investors with their company's news. Please fill out this form to receive the latest information.
Note: The company can only disclose information that is shared in the public domain through press releases, SEC filings, and other public forums. As securities law and industry regulations require, such information will always be shared with all investors simultaneously.