
.png?width=239&height=150&name=NEWTON%20GOLF%20-%20LOGO%20-%20FINAL%20-%20V1%20-%20UPDATE%20-%20WHITE%20(1).png)
NEWTON GOLF COMPANY
Newton Golf Company (Nasdaq: NWTG) is a technology-forward golf company that helps golfers elevate their game. With a growing portfolio of golf products, including putters, golf shafts, golf grips, and other golf-related accessories, the Company’s innovative accomplishments encompass the First Vernier Acuity putter, patented Ultra-Low Balance Point (ULBP) putter technology, weight-forward Center-of-Gravity (CG) design, and pioneering ultra-light carbon fiber putter shafts.
Considering its growth opportunities in golf shaft technologies, the Company expanded its manufacturing business in April of 2022 to develop the advanced Newton brand of premium golf shafts by opening a new shaft manufacturing facility in St. Joseph, MO. The Company intends to manufacture and assemble substantially all products in the United States while expanding into golf apparel and other golf-related product lines to enhance its growth.
The Company’s future expansions may include broadening its offerings through mergers, acquisitions, or internal developments of complementary product lines to its premium brand. The Company currently sells its products through resellers, its websites, Club Champion retail stores, and distributors in the United States, Japan, and South Korea.
Nasdaq: NWTG
NEWTON GOLF - Investors & News: https://www.newtongolfir.com
Nasdaq Profile: https://www.nasdaq.com/market-activity/stocks/nwtg
Headquarters: Camarillo, California
Investor Contact: IR@newtongolfco.com
TALK TO MANAGEMENT
Newton Golf Company is always available to talk to current and potential investors. They're happy to answer any questions you may have and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
Newton Golf Company At-A-Glance
Newton Golf Company designs and manufactures premium-priced golf equipment using physics-based technology to improve player performance. The two main product lines include patented Ultra-Low Balance Point putters featuring high MOI (Moment of Inertia) and Center of Gravity design, along with Newton Motion golf shafts using an innovative elongated bend profile and symmetry 360 design.
Since establishing its Missouri manufacturing facility in 2022, the company has expanded its premium shaft offerings while maintaining U.S.-based production. Distribution channels include direct-to-consumer sales, Club Champion retail stores, and international distributors across the U.S., Japanese, and South Korean markets.
Key Considerations:
Press Releases & Media Coverage
Social Media Updates

Recent LinkedIn Posts
Recent Facebook, Instagram, X, YouTube, TikTok and Pinterest PostsSa
Investor Presentation


To download the Newton Golf Company investor presentation, please fill out the form below.
Stock Chart (Intraday)
Stock Chart (Historical)
Stock Detail
Lead Drug Candidate: Crofelemer

Crofelemer, brand name Mytesi®, is a prescription drug made from the sap of the Croton lechleri tree found in the Amazon rainforest. Indigenous peoples in South America have used this tree sap for centuries to treat a variety of ailments, including diarrhea.
Scientists studied why the sap helped with diarrhea and found it contains chemicals called proanthocyanidins that help normalize fluid secretion in the intestines.
Crofelemer is considered a "first-in-class" drug because it is the first approved drug to work as a gastrointestinal chloride channel modulator (GCCM). The cells lining the intestines have tiny channels that regulate fluid secretion. Crofelemer acts on chloride channels called CFTR and CaCC to reduce the excessive fluid loss into the intestines that causes diarrhea.
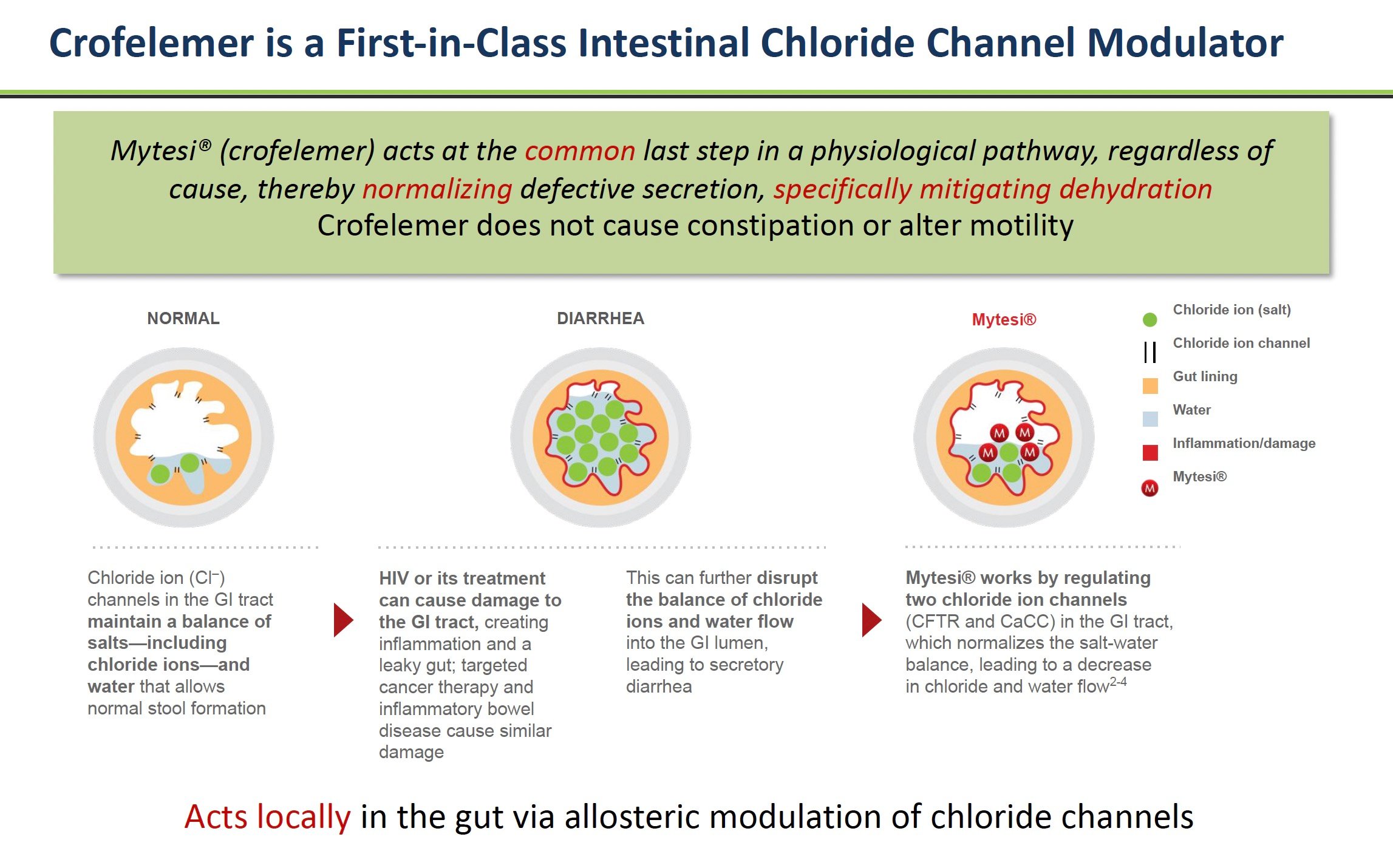
Source: Company Documents
This unique mechanism of action is vital because other antidiarrheal drugs, such as Imodium or loperamide, derived from opioids, work differently by slowing down gut motility. The unintended side-effect of reducing motility is that it can cause constipation. In contrast, crofelemer targets the root cause of secretory diarrhea to return patients to more normal GI function.
After being studied for decades, crofelemer was first approved by the FDA in 2012 to treat noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. Since then, crofelemer has been undergoing rigorous testing in clinical trials to evaluate the drug’s effectiveness for the treatment of diarrhea from other conditions like cancer therapy and rare diseases.
For example, a crofelemer drug called Canalevia-CA1 is now FDA-conditionally approved for the treatment of chemotherapy-induced diarrhea in dogs. Testing in dogs supports potential use in humans for this same indication. Crofelemer is also being studied in rare "orphan" diseases like short bowel syndrome (SBS) and microvillus inclusion disease (MVID) with intestinal failure – two devastating conditions that require patients to be dependent on parenteral (IV) nutrition.
By leveraging its unique mechanism of action normalizing fluid secretion, crofelemer may provide a broadly effective treatment for multiple chronic GI conditions beyond just the treatment of HIV-related diarrhea. More label expansions and partnerships could occur if ongoing studies demonstrate crofelemer's efficacy across additional gastrointestinal diseases.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Important Safety Information About Canalevia - For oral use in dogs only. Not for use in humans. Keep Canalevia-CA1 (crofelemer delayed-release tablets) in a secure location out of reach of children and other animals. Consult a physician in case of accidental ingestion by humans. Do not use in dogs that have a known hypersensitivity to crofelemer. Prior to using Canalevia-CA1, rule out infectious etiologies of diarrhea. Canalevia-CA1 is a conditionally approved drug indicated for the treatment of chemotherapy-induced diarrhea in dogs. The most common adverse reactions included decreased appetite, decreased activity, dehydration, abdominal pain, and vomiting. Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed. It is a violation of Federal law to use this product other than as directed in the labeling. Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-552. See full Prescribing Information at Canalevia.com.
How Crofelemer Works

Crofelemer is a prescription medicine that comes from the sap of a tree sustainably harvested from the Amazon rainforest and works differently than other treatments for gastrointestinal (GI) problems like diarrhea.
With most chronic diarrhea, too much water flows into the GI tract. This action causes loose, watery stools as the extra fluid mixes in. Crofelemer helps normalize water secretion into the intestines.
Inside the lining of the intestines are tiny channels that control the flow of water and salts like chloride into the GI tract. In some diseases, these channels become hyperactive, allowing too much fluid to enter the intestines. This fluid imbalance results in watery diarrhea.
Crofelemer acts directly on two critical intestinal channels called CFTR and CaCC to reduce their hyperactivity. Crofelemer normalizes the amount of fluid secreted into the intestines by calming these overactive channels.
With less extra water flowing into the intestines, stools become more solid, and patients have fewer loose, watery bowel movements.
In contrast, other antidiarrheal drugs like Imodium and loperamide work by slowing down the GI tract. But this opioid approach can cause side effects such as constipation.
Crofelemer treats the root cause of secretory diarrhea – too much fluid secretion into the intestines. It acts locally in the GI tract, normalizing water flow through chloride channel modulation. And it is non-opioid, so it does not cause opioid side effects.
By targeting and modulating the specific chloride channels involved in excess intestinal fluid secretion, crofelemer can effectively reduce chronic diarrhea without slowing overall GI motility like opioids. This novel mechanism of action makes crofelemer well-suited for chronic use in conditions like HIV-associated diarrhea.
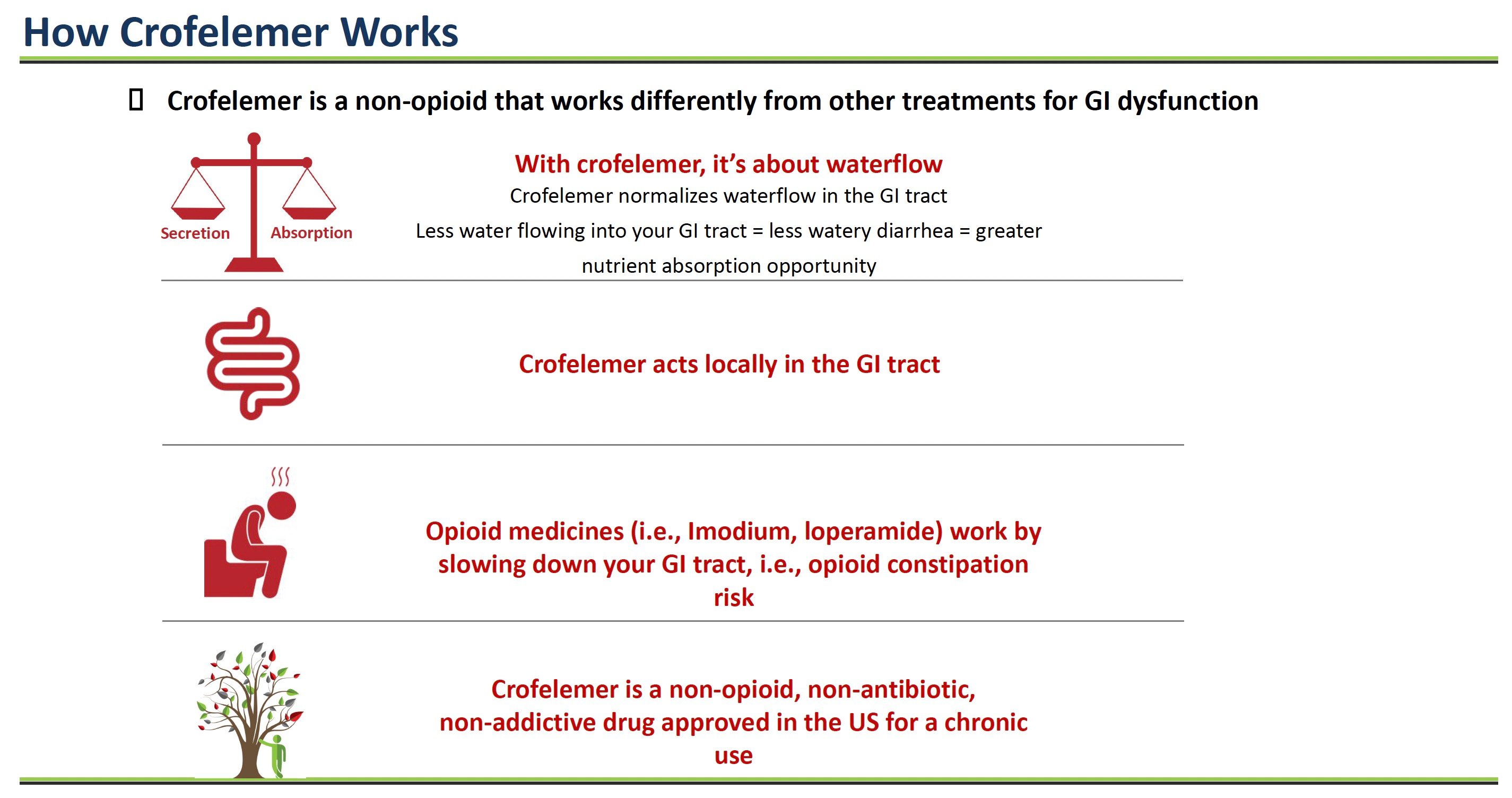
Source: Company Documents
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Jaguar/Napo Product Portfolio – Crofelemer Pipeline

Jaguar Health is developing multiple formulations of its key product, crofelemer, to treat different gastrointestinal diseases. Crofelemer is a prescription drug derived from tree sap that modulates intestinal fluid secretion to alleviate diarrhea. Jaguar has an extensive development pipeline for crofelemer ranging from the initial preclinical research stage to an already approved product. The goal is to expand crofelemer's use across multiple chronic GI conditions.
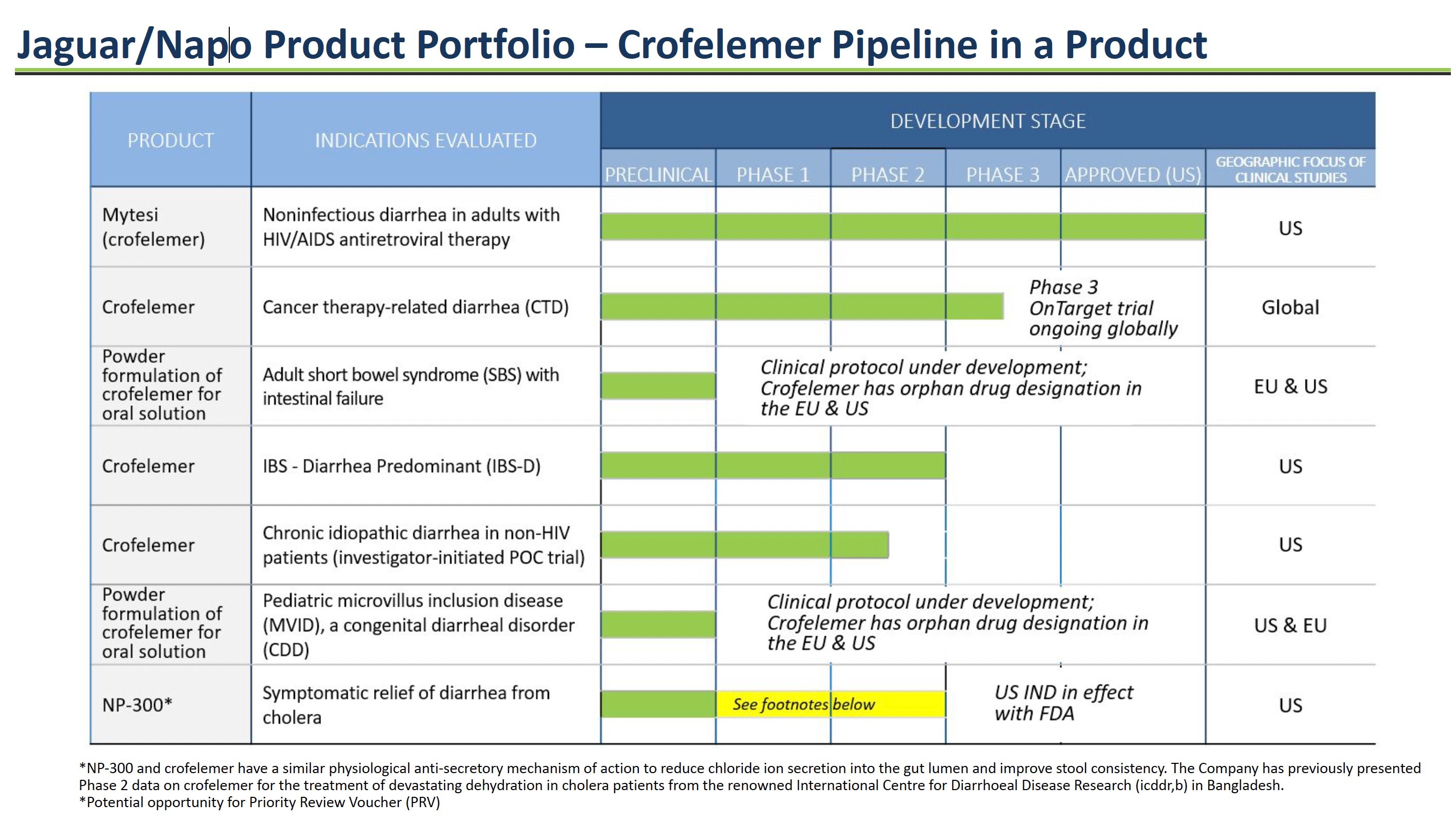
Source: Company Documents
Mytesi® for HIV-Related Diarrhea
Mytesi (crofelemer) is an FDA-approved drug for the treatment of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. It normalizes fluid transport in the intestines to reduce chronic diarrhea. Mytesi was Jaguar's first approved crofelemer product, generating revenue since its launch in 2016.
Crofelemer for Cancer Therapy-related Diarrhea
Jaguar is conducting a Phase 3 clinical trial of crofelemer for cancer therapy-related diarrhea in patients receiving targeted therapies. These cancer agents often damage the gut lining, causing loose stools. Crofelemer could reduce this side effect.
Crofelemer Powder for Rare Orphan Diseases: Short Bowel Syndrome & Microvillus Inclusion Disease
A powder formulation of crofelemer is in development for short bowel syndrome (SBS) and microvillus inclusion disease (MVID) patients with intestinal failure who require IV nutrition. By modulating intestinal fluid, crofelemer may reduce chronic diarrhea in these patients. Orphan-drug incentives could support development. The FDA activated the company’s Investigational New Drug application for crofelemer for the treatment of MVID on August 7, 2023.
Crofelemer for Irritable Bowel Syndrome
Crofelemer has undergone clinical evaluation for patients with diarrhea-predominant IBS. Crofelemer may provide symptomatic relief for chronic loose, watery stools by controlling intestinal fluid secretion.
NP-300 for Cholera Diarrhea
NP-300 is a second-generation anti-secretory antidiarrheal in development for the relief and treatment of moderate-to-severe diarrhea from bacterial, viral, and parasitic infections, including Vibrio cholerae, the bacterium that causes cholera. In September 2023, the FDA activated the company’s Investigational New Drug (IND) application for NP-300 for crofelemer for this indication. NP-300 has a similar mechanism of action as crofelemer but is less costly to produce.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Videos – Jaguar Health: Improving Lives

Please watch the following videos for more information about Jaguar Health and how the company is working to improve lives.
-
From Tree to Bottle
-
December 2021 Discussion About CTD by Lee Schwartzberg, MD, FACP, Professor of Medicine, University of Tennessee Health Science Center, and Napo Pharmaceuticals Scientific Advisory Board (SAB) Member.
-
Lisa Conte Interview with Sandra M. Swain, MD, FACP, FASCO (member of Jaguar/Napo Scientific Advisory Board).
-
Lisa Conte Interview with Dr. Kelly Shanahan, a former clinician and a metastatic breast cancer patient who is now a full-time independent patient advocate and a member of the Jaguar/Napo Scientific Advisory Board.
Source: Company Documents
Global Market Growth Opportunities

Jaguar Health is pursuing a pipeline of crofelemer formulations that span from supportive care for overactive bowel – which includes symptoms such as chronic debilitating diarrhea, GI urgency, and GI incontinence – to potential disease-modifying products in areas of high unmet need. The company’s initiatives represent an expansion into higher-value markets with greater potential to impact patient outcomes and lower the cost of care. The global prescription and OTC gastrointestinal agent market alone is projected to reach $21 billion by 2025, presenting sizable commercial opportunities if Jaguar can successfully develop and differentiate its products.
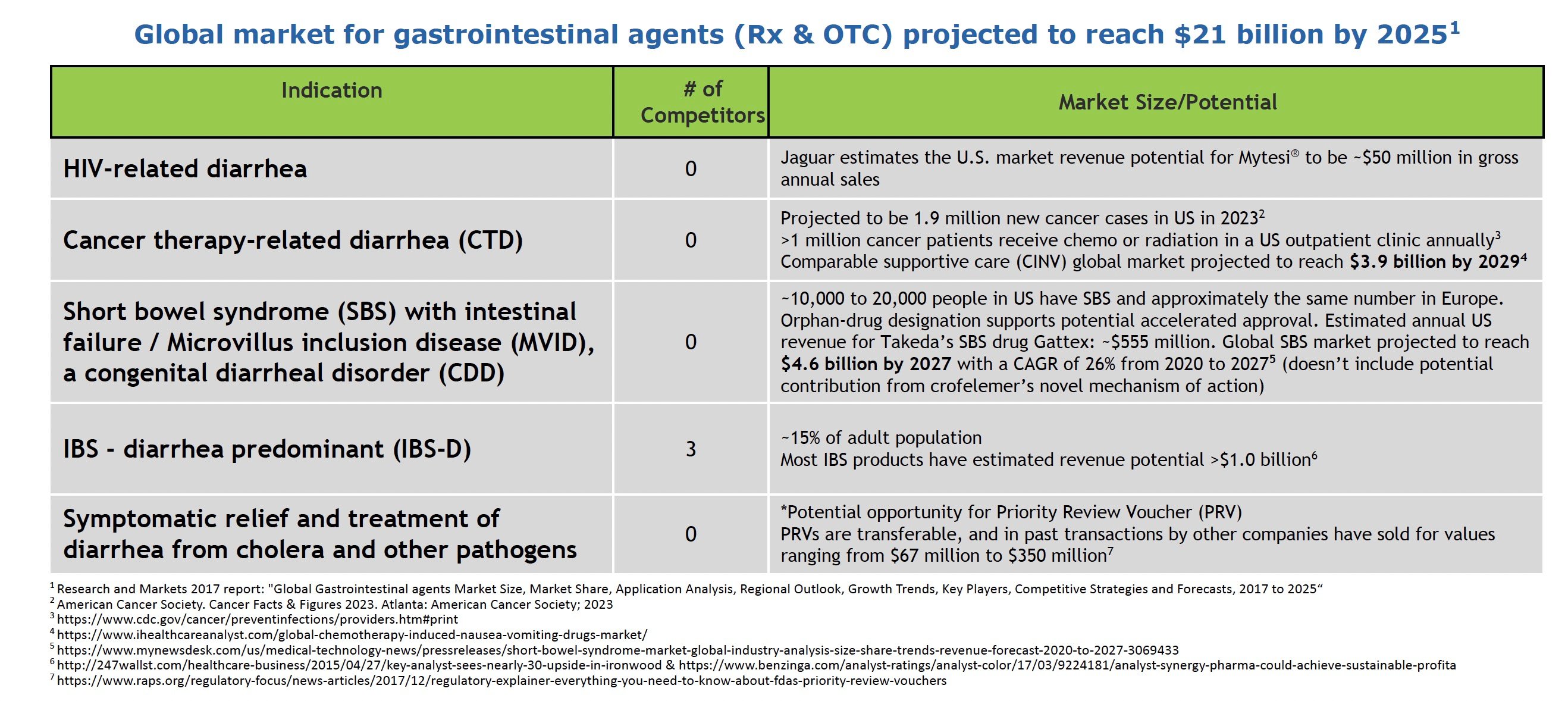
Source: Company Documents
In HIV-related diarrhea, Mytesi is the only FDA-approved treatment. About 70% of HIV/AIDS patients in the US are now over 50 and have lived with the virus for over ten years, often causing chronic gut complications that require symptomatic relief.
For cancer therapy-related diarrhea (CTD), 1.9 million new cancer cases were projected in the US for 2023. Up to 80% of chemotherapy patients experience diarrhea, requiring treatment delays/changes. By preventing CTD, crofelemer could support better adherence and outcomes. The global market for a comparable supportive care indication, chemotherapy-induced nausea and vomiting (CINV), is projected by market research firm iHealthcareAnalyst to reach a value of $3.9 billion by 2029
In short bowel syndrome (SBS) and congenital diarrheal disorders like microvillus inclusion disease (MVID), crofelemer may reduce dependence on parenteral nutrition. About 10,000-20,000 people in the US have SBS. MVID is an ultra-rare disease affecting a couple of hundred patients globally. The global SBS market alone is projected to grow to $4.6 billion by 2027, according to market research firm Vision Research Reports.
For diarrhea-predominant IBS, which impacts an estimated 15% of US adults, crofelemer would target the root cause of chronic watery diarrhea, unlike current treatments. Competitors have estimated peak US sales above $1 billion annually for drugs in this category. Effective relief of IBS-D symptoms remains a major unmet need.
Finally, in infectious diarrhea from pathogens like Vibrio cholerae, the company’s NP-300 drug product candidate may qualify for a Priority Review Voucher (PRV) from the FDA. PRVs are transferable and, in past transactions by other companies, have sold for values ranging from $67 million to $350 million. Additionally, it may meet a global need for affordable infection control in developing markets. In September 2023, the FDA activated the company’s Investigational New Drug (IND) application for NP-300 for crofelemer for this indication.
In summary, Jaguar Health's crofelemer pipeline spans high-value offerings in potential multi-billion-dollar markets where it can fill significant unmet needs related to diarrhea control and intestinal fluid regulation if successfully developed and commercialized.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
2023 Critical Activities to Unlock Jaguar Health's Value Potential

Jaguar Health has laid out several important goals and targets for 2023 through 2024:
A major milestone will be the expected availability in late October 2023 of the primary endpoint data for Napo’s pivotal Phase 3 OnTarget trial of crofelemer for preventative treatment of chemotherapy-induced overactive bowel (CIOB) in adults with cancer on targeted therapy. The primary endpoint looks at the study's main goal – evaluating crofelemer's effectiveness in reducing diarrhea rates. Jaguar Health anticipates being able to present additional data from this pivotal trial data at a medical conference in December 2023.
An independent pilot phase 2 study of crofelemer for the management of neratinib-associated diarrhea in patients with HER2+ early-stage breast cancer indicates that crofelemer, Napo’s FDA-approved drug, may be effective for the management of neratinib-induced diarrhea. The results of the study, which was designed by the study's principal investigator, Jo Chien, MD, and published in the peer-reviewed journal Clinical Breast Cancer, are consistent with the results of a preclinical dog study evaluating the effects of crofelemer in improving diarrhea associated with neratinib, a tyrosine kinase inhibitor (TKI), presented at the American Association for Cancer Research Virtual Annual Meeting II in June 2020.
Jaguar Health's pipeline includes pursuing crofelemer for short bowel syndrome (SBS) and congenital diarrheal disorders (CDD), which both qualify as rare diseases.
Source: Company Documents
Jaguar Health is supporting proof-of-concept clinical trials of crofelemer conducted by independent investigators for SBS and CDD. Proof-of-concept trials can provide early evidence that a drug may be effective. Jaguar Health expects that results from SBS and CDD proof-of-concept studies will be available before the end of 2023 in the first half of 2024.
The company believes the SBS and CDD proof-of-concept data could support expanded patient access to crofelemer through early access programs in specific European countries starting as early as 2024. Early access programs allow patients to receive a medicine before it is fully approved and can generate revenue while also allowing faster access for patients with serious diseases.
Also, in the second half of 2023, Jaguar Health expects the results of a canine study they completed evaluating crofelemer for preventing chemotherapy-related diarrhea in dogs to be published. This canine data could further support crofelemer's use for managing diarrhea from cancer treatments in humans.
Jaguar Health is targeting business development partnerships related to its pipeline and global commercialization efforts in 2023. Partnerships with other companies can help extend crofelemer's reach by licensing rights in certain geographies and disease areas.
Jaguar Health’s major clinical trial readouts, publications, and regulatory milestones spanning multiple programs slated for 2023-2024 all bode well for the company. Meeting these ambitious goals could significantly advance crofelemer toward approvals in cancer therapy-related diarrhea, SBS, and CDD while also expanding real-world evidence and patient access. Jaguar Health aims to execute new partnerships to extend commercial reach globally. Delivering on these key milestones will be critical for Jaguar Health's value and future growth potential.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Important Safety Information About Canalevia - For oral use in dogs only. Not for use in humans. Keep Canalevia-CA1 (crofelemer delayed-release tablets) in a secure location out of reach of children and other animals. Consult a physician in case of accidental ingestion by humans. Do not use in dogs that have a known hypersensitivity to crofelemer. Prior to using Canalevia-CA1, rule out infectious etiologies of diarrhea. Canalevia-CA1 is a conditionally approved drug indicated for the treatment of chemotherapy-induced diarrhea in dogs. The most common adverse reactions included decreased appetite, decreased activity, dehydration, abdominal pain, and vomiting. Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed. It is a violation of Federal law to use this product other than as directed in the labeling. Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-552. See full Prescribing Information at Canalevia.com.
Commercial Rights and Partnerships

Source: Company Documents
Jaguar Health holds global commercial rights to Mytesi® (crofelemer), its FDA-approved drug for the symptomatic relief of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. Jaguar also holds global rights to developing and commercializing crofelemer for multiple possible follow-on indications, including cancer therapy-related diarrhea.
In the animal health area, Jaguar holds the global rights to Canalevia®-CA1, its oral prescription drug FDA conditionally approved for the treatment of chemotherapy-induced diarrhea (CID) in dogs. Jaguar is focused on commercializing Canalevia-CA1 for CID in dogs in the United States.
In Europe, Jaguar has licensed the rights to crofelemer for short bowel syndrome, congenital diarrheal disorders, and HIV-related diarrhea to Jaguar family company Napo Therapeutics. This initiative provides Napo Therapeutics the exclusive rights to develop and commercialize crofelemer in Europe for these indications.
Additionally, Jaguar has partnered with Quadri Pharma for the potential commercialization of Mytesi in select countries in the Middle East and North Africa region. Quadri Pharma has exclusive promotional, distribution, and commercialization rights for Mytesi in these territories, following any necessary regulatory approvals.
Jaguar also has an agreement with Knight Therapeutics for the potential commercialization of Mytesi and other Jaguar prescription products in Canada and Israel. Knight holds exclusive distribution and commercialization rights in these territories.
Overall, Jaguar retains global rights to Mytesi, Canalevia-CA1, and the crofelemer pipeline while also retaining the ability to execute select partnerships to extend commercialization in key international markets. The company’s partnerships give Jaguar control over its core assets while leveraging partners to maximize the global value of its products.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Important Safety Information About Canalevia - For oral use in dogs only. Not for use in humans. Keep Canalevia-CA1 (crofelemer delayed-release tablets) in a secure location out of reach of children and other animals. Consult a physician in case of accidental ingestion by humans. Do not use in dogs that have a known hypersensitivity to crofelemer. Prior to using Canalevia-CA1, rule out infectious etiologies of diarrhea. Canalevia-CA1 is a conditionally approved drug indicated for the treatment of chemotherapy-induced diarrhea in dogs. The most common adverse reactions included decreased appetite, decreased activity, dehydration, abdominal pain, and vomiting. Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed. It is a violation of Federal law to use this product other than as directed in the labeling. Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-552. See full Prescribing Information at Canalevia.com.
Jaguar and Magdalena Biosciences Collaboration

Source: Company Documents
Beyond its core focus, Jaguar Health has invested in an intriguing new venture targeting mental health disorders through natural plant-based prescription drugs. This initiative, known as the Entheogen Therapeutics Initiative (ETI), exemplifies Jaguar Health's long-term vision to leverage its extensive knowledge of ethnobotanical plant medicine to develop innovative therapies across various disease areas.
The ETI is centered around exploring plants with potential mental health benefits, with an initial focus on discovering treatments for depression. This novel program aligns with Jaguar Health's mission to sustainably develop first-in-class medicines from rainforest plants through collaborations with indigenous communities. Jaguar Health's recent formation of the joint venture Magdalena Biosciences crystallizes the ETI strategy into a concrete drug development vehicle targeting major mental health needs.
Origins of Jaguar Health's Mental Health Focus
Jaguar Health's interest in expanding into mental health traces back to the experience of its management team members at the company's predecessor, Shaman Pharmaceuticals. In the 1990s, Shaman conducted extensive global ethnobotanical research, yielding a library of over 2,300 medicinal plants used in traditional healing systems. The team's field studies in Nigeria investigated Native African plants like Picralima nitida, which traditional healers have used to treat psychotic disorders.
When Jaguar Health acquired this plant library from the bankruptcy of Shaman in 2001, it gained a unique asset bridging indigenous medicine and Western pharmaceutical science. Jaguar Health has continued to build upon this library through additional rainforest research. Hence, Jaguar Health possesses unparalleled knowledge of medicinal plants, including many with potential but unexplored effects on the mind and mental health.
The Entheogen Therapeutics Initiative
Building upon this foundation, in 2021, Jaguar Health launched the Entheogen Therapeutics Initiative to formally pursue drug candidates from psychoactive plants for various mental health indications. The term "entheogen" refers to plants that induce spiritual or mystical experiences, many of which have traditional ceremonial uses. Jaguar Health's ETI goes beyond exploring recreational uses, focusing instead on developing rigorously tested prescription drugs for specific medical needs.
The ETI is advised by a Scientific Strategy Team of leading ethnobotanists, physicians, and pharmacologists, including several experts involved in the original Shaman library of plants. This team's knowledge of plant-based medicine and connections with indigenous communities is invaluable for identifying promising new drug candidates. Jaguar Health also chairs an ETI Ethics Board to ensure activities adhere to ethical standards regarding traditional knowledge and fair benefit-sharing.
Initially, the ETI is targeting the discovery of plant-based treatments for mood disorders like depression, anxiety, and PTSD. Depression, in particular, represents a significant unmet medical need, with over 21 million Americans affected each year. Existing antidepressant drugs like SSRIs have limitations in efficacy and side effects, creating demand for new therapeutic approaches. Plants offer a rich starting point for psychiatric drug discovery, given their long use in traditional healing systems.
The ETI has already pinpointed lead compounds from Jaguar Health's library for further investigation. One example is alstonine, a compound derived from the Picralima nitida plant used by traditional healers in Nigeria to treat psychiatric conditions. While still early stage, alstonine could provide a novel treatment mechanism compared to existing antidepressant drugs.
Formation of Magdalena Biosciences Joint Venture
To accelerate ETI's long-term mission, Jaguar Health recently established an exciting joint venture called Magdalena Biosciences, focused specifically on developing plant-based drugs for mental health disorders. Formed in January 2023 in partnership with the Canadian company Filament Health and with backing from One Small Planet, Magdalena Biosciences represents a milestone in Jaguar Health's bold push into mental health.
Magdalena Biosciences is leveraging Jaguar Health's extensive plant library along with Filament Health's drug development expertise. The joint venture has an exclusive license to evaluate plants and extracts from Jaguar Health's collection for specified mental health indications.
The first goal is to identify and optimize plants that may have promise for treating adult ADHD. ADHD is a common disorder affecting over 10 million adults in the U.S., yet existing stimulant treatments have misuse potential. Non-stimulant drugs are limited, creating an opportunity for plant-based alternative prescription drugs with new mechanisms of action and improved safety profiles.
In the future, Magdalena Biosciences plans to pursue additional mood disorders like anxiety, depression, and PTSD. The ultimate vision is to partner with pharmaceutical companies to fully develop and commercialize the venture's botanical drug candidates.
Jaguar Health owns approximately 40% of Magdalena Biosciences, initially valued at $5 million based on One Small Planet's seed investment of $1 million. This ownership stake provides Jaguar Health equity participation in Magdalena Biosciences' long-term success. Jaguar Health also benefits near-term from the $1 million infusion into its plant library and ethnobotanical expertise.
Strategic Context and Implications
Jaguar Health's pursuit of plant-based mental health drugs represents a bold long-term diversification, leveraging the company's unparalleled botanical knowledge and a large library of plants with potential medicinal benefits into new disease areas beyond core gastrointestinal indications. The ETI and Magdalena Biosciences joint venture strategically expands Jaguar Health's pipeline, targeting major conditions like mood disorders and ADHD, which affect millions of patients globally.
The ETI focuses on natural plant compounds that differentiate Jaguar Health's drug candidates from conventional synthetic psychiatric medications. These new drugs may offer improved efficacy, safety, and abuse potential profiles that drive adoption. Additionally, the basis of traditional plant medicine aligns with growing consumer interest in natural remedies over conventional medicines.
Of course, Jaguar Health faces formidable obstacles in proving the safety and efficacy of complex botanical drugs for mental illness. Clinical development timelines are lengthy, costs substantial, and failure rates high. But Jaguar Health is taking a prudent long-term approach through the ETI and Magdalena Biosciences, leveraging its unparalleled experience in ethnobotanical drug discovery.
If ultimately successful, Jaguar Health's novel plant-based therapies could transform the treatment of depression, ADHD, and other psychiatric conditions. This effort would enable Jaguar Health to tap into a multibillion-dollar market and drive significant shareholder value. In the nearer term, the ETI and the Magdalena Biosciences joint venture expand Jaguar Health's portfolio pipeline beyond gastrointestinal medicine, mitigating risk through therapeutic diversification.
Overall, the promise of plant-based mental health prescription drugs makes this an intriguing, if still highly speculative, new frontier for Jaguar Health, warranting close monitoring in the years ahead.
Financial Summary

Jaguar Health, Inc. (NASDAQ: JAGX) is a commercial-stage pharmaceuticals company focused on developing novel proprietary prescription medicines sustainably derived from plants from rainforest areas for people and animals with GI distress, specifically overactive bowel, which includes symptoms such as chronic debilitating diarrhea, GI urgency, and GI incontinence.
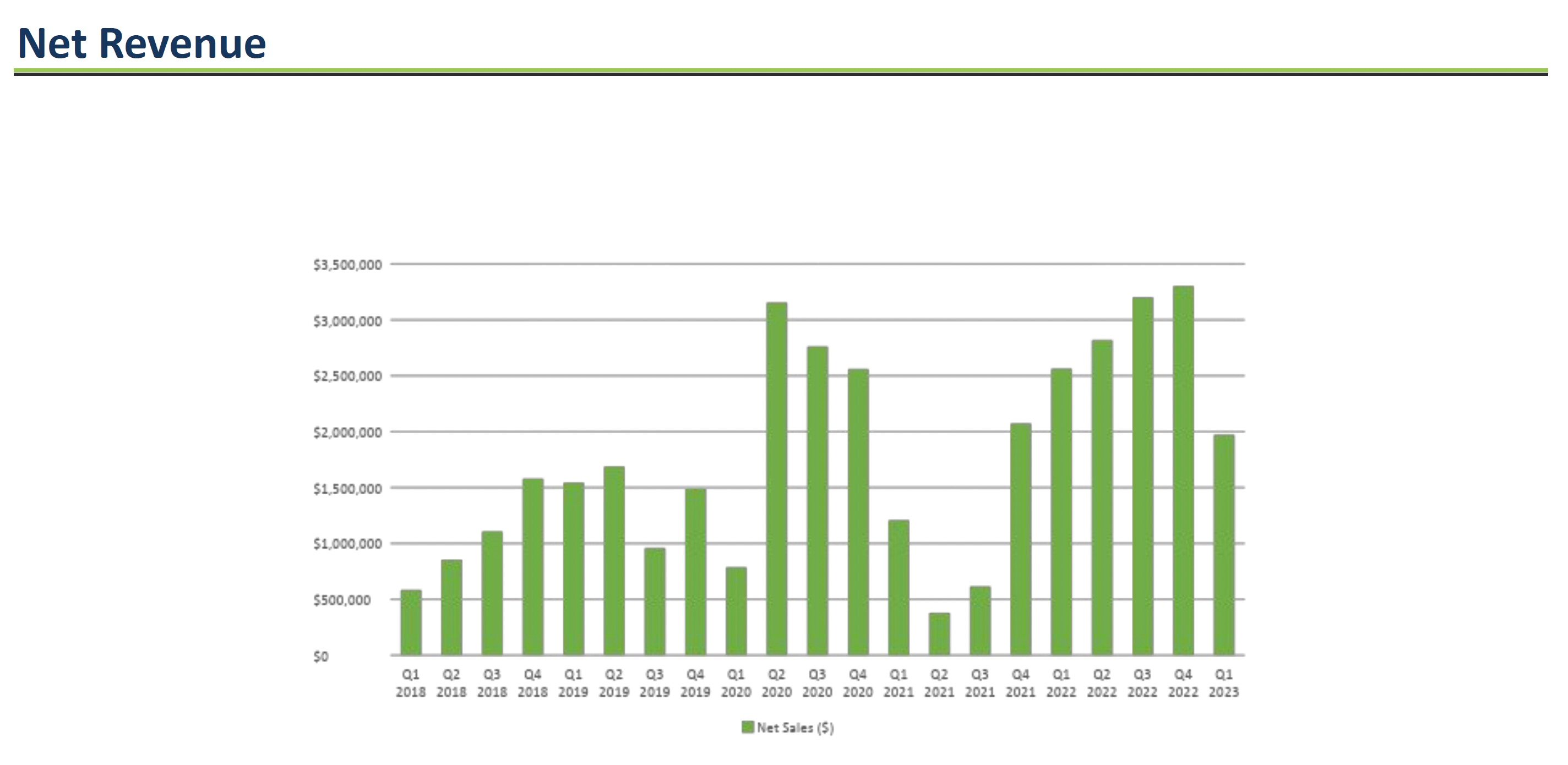
In 2022, Jaguar reported total revenues of $12 million, a significant 175.8% increase compared to revenues of $4.3 million in 2021. The revenue growth was primarily driven by increased sales of their human drug Mytesi® for symptomatic relief of noninfectious diarrhea in HIV/AIDS patients. This growth reflects the company's transition from a wholesaler distribution model to distribution through specialty pharmacies, resulting in lower discounts, rebates, and fees than the prior year. Jaguar also launched sales of their new animal drug Canalevia®-CA1 for chemotherapy-induced diarrhea in dogs in 2022, generating $167,000 in initial revenues.
Source: Company Documents
Despite strong revenue growth, Jaguar continued to report net losses in 2022 of $48.4 million, although this was an 8% improvement from net losses of $52.6 million in 2021. The losses are attributed to high operating expenses related to R&D for Jaguar's pipeline programs, including crofelemer for cancer therapy-related diarrhea and commercialization activities for Mytesi and Canalevia-CA1. Key expense items in 2022 included R&D expenses of $17.6 million, sales and marketing expenses of $8.8 million, and G&A expenses of $17.9 million.
Jaguar is progressing with developing crofelemer for additional indications beyond HIV-related diarrhea, including chemotherapy-induced overactive bowel (CIOB) and inflammatory bowel diseases. Crofelemer is the subject of the OnTarget study, Napo’s ongoing pivotal Phase 3 clinical trial for preventative treatment of CIOB in adults with cancer. Top-line data for the primary endpoint from this trial is expected at the end of October 2023.
The company has received orphan drug designations from the FDA and EMA for crofelemer for two rare diseases: short bowel syndrome (SBS) and microvillus inclusion disease (MVID, a congenital diarrheal disorder (CDD). Jaguar is supporting third-party investigational proof-of-concept studies of crofelemer for these indications that could enable early patient access in Europe, possibly in 2024. Further pipeline programs include NP-300, a second-generation anti-secretory agent for the treatment of moderate-to-severe diarrhea from bacterial, viral, and parasitic infections, including Vibrio cholerae, the bacterium that causes cholera. In September 2023, the FDA activated the company’s Investigational New Drug (IND) application for NP-300 for crofelemer for this indication. NP-300 has a similar mechanism of action as crofelemer but is less costly to produce. Jaguar also formed a joint venture in 2023 with Filament Health, called Magdalena Biosciences, to develop novel plant-based drugs for mental health indications, including ADHD, depression, and anxiety.
In animal health, Jaguar now markets Canalevia-CA1 for the treatment of chemotherapy-induced diarrhea in dogs under FDA conditional approval.
For long-term investors, Jaguar Health represents an early-stage biopharma with high-risk but potentially high reward as it aims to establish a differentiated pipeline of first-in-class plant-based medicines.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Important Safety Information About Canalevia - For oral use in dogs only. Not for use in humans. Keep Canalevia-CA1 (crofelemer delayed-release tablets) in a secure location out of reach of children and other animals. Consult a physician in case of accidental ingestion by humans. Do not use in dogs that have a known hypersensitivity to crofelemer. Prior to using Canalevia-CA1, rule out infectious etiologies of diarrhea. Canalevia-CA1 is a conditionally approved drug indicated for the treatment of chemotherapy-induced diarrhea in dogs. The most common adverse reactions included decreased appetite, decreased activity, dehydration, abdominal pain, and vomiting. Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed. It is a violation of Federal law to use this product other than as directed in the labeling. Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-552. See full Prescribing Information at Canalevia.com.
Analyst Coverage

Jaguar Health is followed by several seasoned biopharma analysts at leading investment banks and research boutiques. This sell-side coverage provides important visibility and insights on Jaguar Health to the institutional investment community.
Source: Company Documents
A Deep and Seasoned Leadership Team

Jaguar Health has assembled an experienced leadership team with decades of combined expertise across pharmaceutical R&D, clinical development, manufacturing, business development, commercialization, legal, and financial operations.
The management team is led by Lisa Conte, the company's founder, president, and CEO, who has over 30 years of experience pioneering plant-based prescription medicines. Other key executives include Pravin Chaturvedi, Ph.D., the company’s Chief Scientific Officer and Chair of the Scientific Advisory Board, and Chief Medical Officer Dr. Darlene Horton, who has over 25 years of experience overseeing clinical development in the biopharma industry. This seasoned leadership team provides Jaguar Health with the strategic vision and operational capabilities to advance the company's pipeline of novel plant-based therapies.
Source: Company Documents
Lisa Conte is the founder, president, and chief executive officer, and a member of the board of directors of Jaguar Health, a commercial-stage pharmaceuticals company committed to discovering, developing, and commercializing plant-based prescription medicines for urgent global health needs. In July 2017, two companies founded by Ms. Conte—Napo Pharmaceuticals, a human-focused pharmaceuticals company, and Jaguar Animal Health, the veterinary-focused licensor of all of Napo’s technology—merged and now comprise Jaguar Health. In 1989, Ms. Conte founded Shaman Pharmaceutical and pioneered plant-based prescription medicine investigation and development for more than 30 years. Ms. Conte is a member of the board of directors of Healing Forest Conservatory, serves on the Editorial Advisory Board of Life Science Leader magazine, and serves on the Leadership Council of Pure Earth. She holds an MS in Physiology and Pharmacology from the University of California, San Diego, and an MBA and AB in Biochemistry from Dartmouth College.
Steven King, Ph.D., has served as Chief of Sustainable Supply, Ethnobotanical Research, and Intellectual Property since 2020. He joined the company in 2002 as Senior Vice President of Sustainable Supply, Ethnobotanical Research, and IP. Before that, he was Vice President of Ethnobotany and Conservation at Shaman Pharmaceuticals. He has a Ph.D. in Biology from the Institute of Economic Botany at the New York Botanical Garden and City University of New York and an MS in Biology from the same institutions.
Pravin Chaturvedi, Ph.D., has been Chief Scientific Officer since 2022 and serves as Chair of the Scientific Advisory Board. He has over 30 years of experience in the pharmaceutical industry and has participated in developing multiple approved drugs. He was previously President and CSO of Napo from 2006-2013. He co-founded several biotech companies, including Scion Pharmaceuticals, IndUS Pharmaceuticals, and Oceanyx Pharmaceuticals. He has a Ph.D. in Pharmaceutical Sciences from West Virginia University and a Bachelor's in Pharmacy from the University of Bombay.
Carol R. Lizak has been Chief Financial Officer since 2021. She joined the company in 2019 as Vice President of Finance and Corporate Controller and was promoted to Chief Accounting Officer and then Senior Vice President of Finance before becoming CFO. She has over 20 years of finance experience, including roles at Zosano Pharma, Quantum Secure, Alexza Pharmaceuticals, and HID Global. She holds an MBA from Pepperdine University Graziadio School of Business and Management and a BS in Business Administration from the University of Santo Tomas.
Karen J. Brunke, Ph.D., brings to Jaguar over 30 years of scientific, operational, clinical, senior executive, and corporate development experience in both large and small biotechnology companies. Dr. Brunke has been primarily responsible for negotiating multiple partnerships and licenses in business and corporate development. Dr. Brunke received her BA degree in Biochemistry and a Ph.D. in Microbiology from the University of Pennsylvania.
Darlene Horton, MD, brings over 25 years of clinical research and development, medical affairs, senior executive, and consulting experience in developing investigational and commercialized biopharmaceutical and drug-device combination products. She has extensive clinical development experience in multiple therapeutic areas. Before joining Napo Pharmaceuticals, she led clinical development and regulatory strategy as CMO at Coherus Biosciences, Itero Biopharmaceuticals, and SMC Biotechnology. Dr. Horton completed her Pediatric Cardiology fellowship and Pediatrics Residency at UCSF. She earned an MD and BS in Microbiology from the University of Florida.
David Sesin, Ph.D., is a pharmaceutical scientist with more than 30 years of experience, from drug discovery to manufacturing. Before serving as Jaguar’s CMO, he was Director of Chemistry and QHSE at Bayer CropScience and Director of Chemistry at AgraQuest, Inc. he spent nine years with Shaman Pharmaceuticals. Dr. Sesin holds a Ph.D. from the University of Utah.
Jonathan S. Wolin, JD, has been Chief of Staff and General Counsel since 2019. Before being promoted, he joined the company in 2018 as Chief Compliance Officer and Corporate Counsel. Previously he was an independent consultant, Chief Administrative Officer at Braden Partners, and Chief Compliance Officer at Natera and Braden Partners, among other roles. He holds a JD from The Catholic University of America Columbus School of Law, an MBA from The George Washington University School of Business, and a BS in Accounting from the University of Maryland
Ian Wendt has been Chief Commercial Officer since 2020 after joining as VP of Commercial Strategy in 2019. He has over 15 years of experience in pharmaceutical and biotech sales, marketing, and operations at companies including Gilead Sciences, Boehringer Ingelheim, and Roxane Laboratories. He has an MBA from Dalhousie University in Nova Scotia and a BSc from Acadia University.
Company Overview

Newton Golf Company (Nasdaq: NWTG) was founded in 2018 by industry veterans Steve Sacks and Rich Parente to bring a physics-driven approach to premium golf equipment. Taking inspiration from Sir Isaac Newton’s principles of motion and gravity, the company’s rebranding as NEWTON underscores its commitment to scientifically engineered products that provide players with enhanced precision and control.

Source: Company Documents
Although relatively young, the company has achieved several key milestones. Early on, it secured a South Korean distributor to reach one of the world’s largest golf markets and launched innovative sales programs for golf pros and custom fitters to drive putter sales. During the COVID-19 pandemic, NEWTON introduced a virtual fitting program to help golfers select equipment remotely. In August 2023, the company went public, raising $11.6 million to support ongoing growth initiatives. NEWTON has also continued to expand its product offerings with the Newton Motion Driver shaft, followed closely by the Newton Motion Fairway Woods shaft.
NEWTON’s portfolio is designed to maximize performance through physics-based innovation. The Series 02 DRAC and Series 54MC putters utilize the company’s patented Ultra-Low Balance Point (ULBP) technology, which shifts weight toward the putter head to promote a smoother, pendulum-like stroke that improves stability and alignment. Independent testing shows that this design helps golfers achieve more accurate putts and better impact. In its shaft lineup, the Newton Motion series features an elongated bend profile and Symmetry360 technology, which provides even flex across the shaft length, increasing shot accuracy and distance with less effort. The shafts have also gained traction on the professional circuit, with several Champions Tour players adopting Newton Motion technology.
To support these innovations, NEWTON expanded its U.S. manufacturing capabilities in April 2022 by opening a production facility in St. Joseph, Missouri. This facility allows the company to maintain strict quality control and reduce supply chain risks. With a growing distribution network that includes all 126 Club Champion stores nationwide and partnerships with distributors in Japan and South Korea, NEWTON is solidifying its position in the high-end golf equipment market. Plans for further expansion include entering the golf apparel and accessories market, with potential growth opportunities through strategic acquisitions and partnerships.
 Source: Company Documents
Source: Company Documents
Technical Innovation: Physics-Driven Design

At the core of NEWTON GOLF’s approach is a commitment to designing golf equipment based on the principles of physics, not gimmicks. This science-driven philosophy has led to the development of products that harness advanced engineering to deliver measurable performance benefits. One of the standout features of NEWTON’s putter line is its patented Ultra-Low Balance Point (ULBP) technology, which repositions the balance point within five inches of the putter head—significantly lower than traditional designs. By concentrating more weight toward the head, ULBP promotes a pendulum-like stroke that reduces hand and wrist tension, helping golfers achieve a smoother, more controlled movement. The result, confirmed through independent testing, is a putter that naturally improves accuracy by maintaining a stable, squared face at impact, translating into a more reliable roll and better distance control.
The Newton Motion shafts are another example of physics at work in NEWTON’s product designs. Each shaft is crafted using a unique elongated bend profile that optimizes the shaft’s flex along a greater portion of its length, an engineering choice that enhances energy transfer from the golfer’s swing to the ball. This construction not only increases clubhead speed but also reduces the physical effort required, allowing players to generate longer shots with less strain. To further improve performance, the Newton Motion shafts use Symmetry360 technology, which ensures that the shaft flexes uniformly in all directions. This symmetry allows for consistent performance regardless of the club’s orientation, giving players more predictable ball flight and greater shot accuracy.
Beyond symmetry, NEWTON has incorporated a Variable Bend Profile in its Newton Motion shafts, a feature designed to adapt the shaft’s bend and torque to the unique demands of different swing speeds. For golfers with slower swings, more torque is built into the shaft to enhance flexibility, while higher swing speeds benefit from reduced torque, allowing for more stability and control. This tailored approach creates a custom-fit feel without the need for individualized shaft adjustments, accommodating a range of players and swing types in a single, scientifically engineered design.
An independent study conducted by The Golf Lab in Canada evaluated the performance of Sacks Parente’s putters using a Gears full-body optical motion tracking system. Fifteen golfers with handicaps between 8 and 15 tested Sacks Parente’s Ultra-Low Balance Point (ULBP) putters alongside their own putters, each completing 82 putts with both. The Gears system captured 600 images per putt, providing comprehensive data on alignment, impact angle, club path, and speed control. Results showed a 56% increase in successful putts when using Sacks Parente putters, with make percentages rising from 39% to 62%. Participants demonstrated improved alignment, squared impact, and more consistent club path and speed control, highlighting the ULBP technology’s impact on putter performance and supporting NEWTON’s claims of enhanced putting precision. (See additional details at Golf Lab Independent Study)

Source: Company Documents
Independent Study by The Golf Lab 
Setup
-
15 volunteers with golf handicaps between 8 – 15
-
Each golfer given 82 putts with their own putter and then 82 with a Gravity ULBP putter that they never used before
-
The Golf Lab (independent entity) used its Gears full-body optical motion tracking system to provide “hard data”
-
Gears system measures the putting swing as it unfolds
-
600 images per putt are generated and analyzed in less than a second
Results
-
Gravity putters were empirically proven to:
-
Make more putts
-
Offer better aim/alignment
-
Improve squared angle at impact
-
Improve club path during swing
-
Improve speed control by being naturally easier to align, providing a more consistent stroke/speed control, better club path, and squaring the putter at impact
-
-
56% Increase in number of putts made
-
On average, 32/82 putts were made by the golfers using their own putter vs. 50/82 made with the Gravity putter
-
Make percentage increased from 39% to 62%
-
NEWTON’s commitment to physics-driven design is further supported by its investment in cutting-edge manufacturing capabilities. The company’s production facility in St. Joseph, Missouri, is equipped with proprietary tools and processes that enable precision manufacturing of the Newton Motion shafts. By leveraging technologies such as computer-aided design, finite element analysis, and robotic testing, NEWTON ensures each product meets rigorous performance standards. This facility also allows NEWTON to maintain tight quality control and consistency, solidifying its position as a pioneer in creating golf equipment that combines scientific principles with premium craftsmanship.

Source: Company Documents
Product Portfolio

NEWTON GOLF designs a range of premium-priced golf equipment, specifically engineered to deliver measurable performance improvements. Their product lineup includes high-tech putters and the Newton Motion line of shafts, each developed with proprietary technologies to enhance shot precision, distance, and control.
Putters (Average Selling Prices: $450)
NEWTON GOLF’s putters are known for their patented Ultra-Low Balance Point (ULBP) technology, which repositions the balance point closer to the putter head, creating a pendulum-like motion that is easier to control. This unique design helps players maintain consistent alignment and accuracy throughout their strokes. Key Newton Gravity Putter models include:
1. The DRAC—A mallet-style putter with an advanced weight-forward design to provide enhanced stability and precision. The DRAC is made of precision-milled 6061 aluminum and 100 grams of tungsten in the front of the putter body in the heel and toe and is Gravity's highest (Moment of Inertia) MOI putter.
2. PRISM—A patented, precision-milled putter that naturally improves stroke and tightens putt dispersion. The PRISM is a high-MOI putter that maximizes forgiveness on off-center hits, promoting straighter putts.
3. The DEUCE—The DEUCE is a wide-body anser putter with a blade-like look. It is easy to aim, designed to fit all stroke types, and has two hosel locations.
4. The CLASSIC—The Classic is made with precision-milled 1018 carbon steel and a modern ceramic coating finish. It features a newly designed hosel for enhanced performance and feel and is a traditional anser-style putter.
5. The DUKE—Inspired by PGA Tour Champions player Ken Duke, the Duke putter has a precision-milled 1018 carbon steel body with tungsten weighting. It is a classic-style putter with a shorter blade length and a half-mallet back.

Source: Company Documents
Newton Motion Replacement Shafts (Average Selling Prices: $250 - $275)
The Newton Motion shafts are designed to improve swing efficiency and enhance distance while maintaining a smooth, controlled shot experience. These shafts feature several proprietary innovations:
1. Elongated Bend Profile – This feature distributes the flex along a larger portion of the shaft’s length, creating a smoother swing and increasing energy transfer to the ball. This design choice can boost clubhead speed and reduce the effort needed for longer shots.
2. Symmetry360 Technology ensures consistent flex and torque across all shaft orientations, giving players predictable ball flight and greater shot reliability.
3. Variable Bend Profile – Tailors the shaft’s torque and flex to suit different swing speeds, making it adaptable for players with varying swing strengths. Golfers with slower swings benefit from additional flex, while those with faster swings experience greater control and stability.
The shafts are available in various flex levels to suit individual player needs and are manufactured at NEWTON’s U.S.-based facility in St. Joseph, Missouri, ensuring high standards of quality control.

Source: Company Documents
Patents & Intellectual Property Protection

NEWTON GOLF strongly emphasizes protecting its intellectual property (IP) to maintain a competitive edge in the golf equipment industry. The company’s innovative designs are safeguarded through a combination of patents and trademarks, ensuring that competitors cannot easily replicate their proprietary technologies.
Patents and Proprietary Technologies
One of NEWTON GOLF’s cornerstone innovations is the Ultra-Low Balance Point (ULBP) technology, which repositions the balance point closer to the putter head to promote a pendulum-like stroke. This design is protected under U.S. Patent No. 8,608,586, granted in 2013. The company also holds a patent that covers the ornamental aspects of its putter designs, further strengthening its IP portfolio. In 2020, NEWTON GOLF secured a patent for its magnesium face technology, which replaces traditional aluminum inserts with magnesium to reduce weight and enhance performance.
The company's technology is described in the 90-second video clip below, in which Chief Technology Officer Aki Yorihiro explains what ULBP is and how it improves putting.
Source: Company Documents
Inventors and Company Founders
The primary inventors behind these technologies are company co-founders Richard E. Parente and Steven Sacks. Richard Parente, an original co-founder and the first President of Callaway Golf in 1982, brings extensive experience in golf club design. Steven Sacks began his career in the golf industry in the 1970s, contributing decades of expertise.
Trademarks & IP Strategy
NEWTON GOLF also protects its brand identity through registered trademarks. The company’s name, logos, and product names are trademarked to prevent unauthorized use and to maintain brand integrity in the marketplace. With multiple patents and a robust trademark portfolio, NEWTON GOLF protects its innovations legally. This strategy not only safeguards the company’s competitive position but also provides confidence that competitors cannot easily duplicate the proprietary aspects of its products.
 Source: Patents.Google.Com
Source: Patents.Google.Com
The golf equipment market is highly competitive. In the premium putter segment, the company faces competition from major golf equipment brands such as TaylorMade, Ping, and Acushnet, which includes Scotty Cameron and Titleist brands. Together, these competitors command a significant share of the putter market, with Acushnet and Callaway (Odyssey/Toulon) alone holding approximately 60% of the global market.
In the golf shaft market, Sacks Parente competes with established manufacturers like Fujikura Composites, Mitsubishi Chemical (MRC), Graphite Design, UST Mamiya, and Project X. These companies dominate the market with advanced materials and high-performance shaft technologies.
The company's commitment to proprietary technology, including its Ultra-Low Balance Point (ULBP) putter design and Newton Motion shafts, aims to differentiate its products in a crowded market by offering innovations grounded in physics-driven design principles that competitors cannot easily replicate.
Source: Company Documents
Manufacturing & Operations

In April 2022, NEWTON GOLF expanded its manufacturing capabilities by opening a shaft production facility in St. Joseph, Missouri. This facility focuses on producing the Newton Motion line of golf shafts, allowing the company to maintain strict quality control and reduce reliance on overseas suppliers. By manufacturing domestically, NEWTON GOLF ensures consistency in product quality and mitigates potential supply chain disruptions.
The company’s putters are manufactured in Camarillo, California, further emphasizing its commitment to U.S.-based production. This approach not only supports local economies but also aligns with the company’s goal of producing premium golf equipment with meticulous attention to detail.
Advanced Manufacturing Processes
NEWTON GOLF employs advanced manufacturing techniques to produce its innovative products. Computer-aided design (CAD) software and precision machining ensure that each component meets exact specifications. The company’s proprietary Ultra-Low Balance Point (ULBP) technology in putters and the elongated bend profile in Newton Motion shafts are the results of these sophisticated processes.

Source: Company Documents
Quality Control and Testing
To maintain high standards, NEWTON GOLF implements rigorous quality control measures throughout the manufacturing process. Each product undergoes thorough testing to ensure it meets performance criteria. This commitment to quality is evident in the positive feedback from professional golfers who have adopted NEWTON GOLF’s equipment.
Supply Chain and Distribution
By centralizing manufacturing within the United States, NEWTON GOLF effectively manages its supply chain, reducing lead times and ensuring timely delivery to retailers and customers. The company distributes its products through various channels, including direct-to-consumer sales via its website, partnerships with retailers like Club Champion, and international distributors in Japan and South Korea. This integrated approach to manufacturing and operations positions NEWTON GOLF to deliver high-quality, innovative golf equipment to a global market while maintaining control over production and quality assurance.
Market Presence & Growth Opportunity

NEWTON GOLF, a premium golf equipment company, is strategically positioned in the high-end segment of the golf equipment market, estimated at $10.2 billion globally and $5.3 billion in the U.S. alone. This market includes a growing demand for high-performance, science-driven products like NEWTON’s putters and shafts, which integrate proprietary technology to improve control, accuracy, and distance. In the U.S., approximately 25.6 million people play on golf courses, and with an additional 15.5 million engaged in off-course golf activities, NEWTON is well-placed to capture market interest both on and off the course.
Source: Company Documents
The company’s international expansion efforts have already gained traction, with a distribution partnership in South Korea providing a solid presence in one of the world’s top three golf markets. South Korean golfers are known to spend more per capita on golf equipment and apparel than any other country, making this a lucrative market for NEWTON’s premium products. Japan, the second-largest golf market globally, is also a focus for expansion, positioning NEWTON GOLF for increased international revenue.

Source: Company Documents
Domestically, NEWTON GOLF has recently expanded its retail footprint by placing its products in all 126 Club Champion stores nationwide, broadening its accessibility to U.S. consumers through this respected custom club-fitting chain. Direct-to-consumer (DTC) sales also represent a significant aspect of the company’s growth strategy. NEWTON builds its online presence through targeted digital marketing campaigns across platforms like Google, Meta, Amazon, and Shopify, directly reaching golf enthusiasts and supporting rapid sales growth.
NEWTON GOLF has built a strong presence on professional golf tours, particularly among PGA TOUR Champions players who value the performance advantages of Newton Motion shafts. More than 30 Tour pros, including golf legends like John Daly, Mark O’Meara, Colin Montgomerie, and Doug Barron have chosen these shafts for their reliability and technological benefits, showcasing NEWTON’s commitment to high-quality, scientifically engineered products.

Source: Company Documents
To further encourage professional adoption, NEWTON launched the Weekly Motion Driver Performance Pool program in 2024. This initiative rewards PGA TOUR Champions players who use Newton Motion shafts and secure top finishes in tournaments, offering them financial incentives for their achievements. The program has drawn participation from renowned players, including Doug Barron, Clark Dennis, Ken Duke, Fred Funk, Tim Petrovic, Wes Short, Duffy Waldorf, and Colin Montgomerie, reinforcing NEWTON’s growing influence among top-tier professionals.
Initiatives Implemented in 2024 to Accelerate Growth
-
Expanded Newton Motion product line beyond drivers – now includes fairway woods
-
Newton Motion Driver shaft product experiencing favorable momentum and interest across the board
-
-
Expanded availability of putters and Newton Motion shafts into all 126 Club Champion stores nationwide
-
Introduced Incentive program for golf pros, custom club fitters, and golf instructors
-
Foster additional point-of-sales outlets that can drive revenue growth and build long-term relationships
-
-
Aggressive digital marketing campaigns on Google, Meta, Amazon and Shopify
-
High return on ad spend
-
-
Signed Champion’s Tour professional golfers to Newton Motion shaft sponsorships
Looking ahead, NEWTON GOLF plans to expand its offerings to include golf apparel and accessories, further enhancing its position as a comprehensive, premium golf brand. Future growth may also come from strategic acquisitions and partnerships, allowing NEWTON to broaden its product line in complementary areas. Additionally, with the Newton Motion shaft line rapidly gaining adoption among Champions Tour players, NEWTON intends to increase its tour presence with an eye on the PGA and LPGA circuits, further strengthening its brand visibility and market reach among professional and amateur golfers.

Source: Company Documents
Management & Board

NEWTON GOLF is led by a team with deep expertise across engineering, management, sales, and technology. This diverse experience fuels the company’s mission to bring cutting-edge, science-based innovations to the golf industry.
Dr. Greg Campbell, Executive Chairman
Dr. Campbell has served as NEWTON’s Executive Chairman since 2018. He is also the CEO of V-Grid Energy Systems, a California start-up focused on transforming agricultural waste into renewable energy and bio-carbon. With nearly 40 years in advanced technology and product development, Dr. Campbell brings a wealth of experience, having previously led two companies to public offerings. He holds a Ph.D. in Electrical and Electronics Engineering from UCLA and a BA/MA in Engineering from Cambridge University. Before NEWTON, he managed a $1.2 billion division as SVP & GM for Lam Research.
Ryan Stearns, Chief Financial Officer
Ryan Stearns has been the Chief Financial Officer of NEWTON since January 2025, bringing over two decades of financial leadership experience across public, private, and international organizations. Before joining the company, he served as CFO at JTB Strategic Services and Medical Innovations, overseeing complex financial operations and strategic growth initiatives. Stearns holds a Bachelor of Arts in Business Administration with a focus on Accounting and Finance from the University of Washington’s Foster School of Business, where he was recognized as the “Outstanding Student of Finance,” and earned a Master’s Certificate in Private Capital Markets from Pepperdine Graziadio Business School.
Akinobu Yorihiro, Director, Chief Technology Officer
Akinobu "Aki" Yorihiro, a co-founder of NEWTON GOLF, serves as Director and Chief Technology Officer. Previously holding roles as Chief Legal Officer and CFO, Yorihiro oversees the development of NEWTON’s innovative product designs. He is also chairman of the board of Nippon Xport Ventures. His extensive experience includes leadership positions with Yoshimoto Entertainment USA and Bellrock Media, as well as a corporate M&A partnership at Bingham McCutchen LLP, where he managed cross-border transactions. Yorihiro earned his B.A. in Economics and Mathematics from Claremont McKenna College and his J.D. from Georgetown University Law Center.
In addition to Dr. Campbell and Mr. Yorihiro, the company's Board of Directors includes leaders with diverse backgrounds in professional golf, financial services, and product development:
• Dottie Pepper: A 17-time LPGA Tour winner and major champion, Pepper has extensive experience in professional golf and media, including roles with CBS Sports and as a Director with the PGA of America.
• Brett Hoge: Senior Managing Director at Truist Investment Services, Inc., with over two decades in financial services and numerous industry recognitions.
• Jane Casanta: Former Director of Marketing at Acushnet (Titleist), where she drove significant sales growth in gloves and accessories over a 26-year career.
Financial Overview

Newton Golf Company has reported notable growth in recent quarters, primarily driven by the launch of new products. The company’s Newton Motion Driver and Fairway Woods shafts, introduced in late 2023 and early 2024, respectively, significantly accelerated revenue, resulting in a nearly 750% increase in the first half of 2024 compared to the same period in 2023. This rapid growth underscores the market’s positive reception of these physics-driven shafts designed to enhance performance with improved accuracy and distance.
Operationally, Newton Golf has made strategic moves to secure a more resilient supply chain. Establishing an in-house manufacturing facility in Missouri has enabled the company to control production quality and reduce risks associated with overseas manufacturing. The company leverages patented putter designs and proprietary manufacturing processes to maintain competitive advantages, focusing primarily on direct-to-consumer (DTC) channels for expansion.
With expanding distribution in the U.S. and strong partnerships in Japan and South Korea—two of the largest golf equipment markets globally—Newton Golf Company anticipates substantial international sales growth. The company has also recently broadened its presence across all 126 Club Champion stores in the U.S., reinforcing its multi-channel strategy. Expansion into premium golf apparel and accessories is planned, with potential growth through strategic partnerships and acquisitions to enhance its product offerings and strengthen its brand in the high-end golf equipment market.
As of October 2024, the company had a cash reserve of $1.6 million and no debt, positioning Newton Golf Company to pursue growth opportunities. The company’s insiders own a substantial 47% of shares, indicating a firm commitment from its leadership toward driving long-term value for shareholders.
Investment Highlights
-
Recent introduction of Newton Motion Driver shaft and Newton Motion Fairway Woods shaft has accelerated revenue growth by ~750% in 1H24 vs. 1H23
-
Independent lab testing offers proof that Newton Gravity putters help golfers make more putts
-
In-house manufacturing facility in Missouri, patented putters, and proprietary manufacturing processes for new shaft business should reduce the supply chain risks and ensure quality control
-
Rapid multi-year revenue growth expected primarily from product launches of Company’s putters and line of Newton Motion shafts, primarily via DTC channels
-
Recently expanded into all 126 Club Champion stores nationwide
-
Japanese and Korean distributor already in place that provides exposure to the world’s #2 and #3 golf markets → significant international sales grow expected
Source: Company Documents
Lead Drug Candidate: Crofelemer

Crofelemer, brand name Mytesi®, is a prescription drug made from the sap of the Croton lechleri tree found in the Amazon rainforest. Indigenous peoples in South America have used this tree sap for centuries to treat a variety of ailments, including diarrhea.
Scientists studied why the sap helped with diarrhea and found it contains chemicals called proanthocyanidins that help normalize fluid secretion in the intestines.
Crofelemer is considered a "first-in-class" drug because it is the first approved drug to work as a gastrointestinal chloride channel modulator (GCCM). The cells lining the intestines have tiny channels that regulate fluid secretion. Crofelemer acts on chloride channels called CFTR and CaCC to reduce the excessive fluid loss into the intestines that causes diarrhea.

Source: Company Documents
This unique mechanism of action is vital because other antidiarrheal drugs, such as Imodium or loperamide, derived from opioids, work differently by slowing down gut motility. The unintended side-effect of reducing motility is that it can cause constipation. In contrast, crofelemer targets the root cause of secretory diarrhea to return patients to more normal GI function.
After being studied for decades, crofelemer was first approved by the FDA in 2012 to treat noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. Since then, crofelemer has been undergoing rigorous testing in clinical trials to evaluate the drug’s effectiveness for the treatment of diarrhea from other conditions like cancer therapy and rare diseases.
For example, a crofelemer drug called Canalevia-CA1 is now FDA-conditionally approved for the treatment of chemotherapy-induced diarrhea in dogs. Testing in dogs supports potential use in humans for this same indication. Crofelemer is also being studied in rare "orphan" diseases like short bowel syndrome (SBS) and microvillus inclusion disease (MVID) with intestinal failure – two devastating conditions that require patients to be dependent on parenteral (IV) nutrition.
By leveraging its unique mechanism of action normalizing fluid secretion, crofelemer may provide a broadly effective treatment for multiple chronic GI conditions beyond just the treatment of HIV-related diarrhea. More label expansions and partnerships could occur if ongoing studies demonstrate crofelemer's efficacy across additional gastrointestinal diseases.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Important Safety Information About Canalevia - For oral use in dogs only. Not for use in humans. Keep Canalevia-CA1 (crofelemer delayed-release tablets) in a secure location out of reach of children and other animals. Consult a physician in case of accidental ingestion by humans. Do not use in dogs that have a known hypersensitivity to crofelemer. Prior to using Canalevia-CA1, rule out infectious etiologies of diarrhea. Canalevia-CA1 is a conditionally approved drug indicated for the treatment of chemotherapy-induced diarrhea in dogs. The most common adverse reactions included decreased appetite, decreased activity, dehydration, abdominal pain, and vomiting. Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed. It is a violation of Federal law to use this product other than as directed in the labeling. Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-552. See full Prescribing Information at Canalevia.com.
How Crofelemer Works

Crofelemer is a prescription medicine that comes from the sap of a tree sustainably harvested from the Amazon rainforest and works differently than other treatments for gastrointestinal (GI) problems like diarrhea.
With most chronic diarrhea, too much water flows into the GI tract. This action causes loose, watery stools as the extra fluid mixes in. Crofelemer helps normalize water secretion into the intestines.
Inside the lining of the intestines are tiny channels that control the flow of water and salts like chloride into the GI tract. In some diseases, these channels become hyperactive, allowing too much fluid to enter the intestines. This fluid imbalance results in watery diarrhea.
Crofelemer acts directly on two critical intestinal channels called CFTR and CaCC to reduce their hyperactivity. Crofelemer normalizes the amount of fluid secreted into the intestines by calming these overactive channels.
With less extra water flowing into the intestines, stools become more solid, and patients have fewer loose, watery bowel movements.
In contrast, other antidiarrheal drugs like Imodium and loperamide work by slowing down the GI tract. But this opioid approach can cause side effects such as constipation.
Crofelemer treats the root cause of secretory diarrhea – too much fluid secretion into the intestines. It acts locally in the GI tract, normalizing water flow through chloride channel modulation. And it is non-opioid, so it does not cause opioid side effects.
By targeting and modulating the specific chloride channels involved in excess intestinal fluid secretion, crofelemer can effectively reduce chronic diarrhea without slowing overall GI motility like opioids. This novel mechanism of action makes crofelemer well-suited for chronic use in conditions like HIV-associated diarrhea.

Source: Company Documents
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Jaguar/Napo Product Portfolio – Crofelemer Pipeline

Jaguar Health is developing multiple formulations of its key product, crofelemer, to treat different gastrointestinal diseases. Crofelemer is a prescription drug derived from tree sap that modulates intestinal fluid secretion to alleviate diarrhea. Jaguar has an extensive development pipeline for crofelemer ranging from the initial preclinical research stage to an already approved product. The goal is to expand crofelemer's use across multiple chronic GI conditions.

Source: Company Documents
Mytesi® for HIV-Related Diarrhea
Mytesi (crofelemer) is an FDA-approved drug for the treatment of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. It normalizes fluid transport in the intestines to reduce chronic diarrhea. Mytesi was Jaguar's first approved crofelemer product, generating revenue since its launch in 2016.
Crofelemer for Cancer Therapy-related Diarrhea
Jaguar is conducting a Phase 3 clinical trial of crofelemer for cancer therapy-related diarrhea in patients receiving targeted therapies. These cancer agents often damage the gut lining, causing loose stools. Crofelemer could reduce this side effect.
Crofelemer Powder for Rare Orphan Diseases: Short Bowel Syndrome & Microvillus Inclusion Disease
A powder formulation of crofelemer is in development for short bowel syndrome (SBS) and microvillus inclusion disease (MVID) patients with intestinal failure who require IV nutrition. By modulating intestinal fluid, crofelemer may reduce chronic diarrhea in these patients. Orphan-drug incentives could support development. The FDA activated the company’s Investigational New Drug application for crofelemer for the treatment of MVID on August 7, 2023.
Crofelemer for Irritable Bowel Syndrome
Crofelemer has undergone clinical evaluation for patients with diarrhea-predominant IBS. Crofelemer may provide symptomatic relief for chronic loose, watery stools by controlling intestinal fluid secretion.
NP-300 for Cholera Diarrhea
NP-300 is a second-generation anti-secretory antidiarrheal in development for the relief and treatment of moderate-to-severe diarrhea from bacterial, viral, and parasitic infections, including Vibrio cholerae, the bacterium that causes cholera. In September 2023, the FDA activated the company’s Investigational New Drug (IND) application for NP-300 for crofelemer for this indication. NP-300 has a similar mechanism of action as crofelemer but is less costly to produce.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Videos – Jaguar Health: Improving Lives

Please watch the following videos for more information about Jaguar Health and how the company is working to improve lives.
-
From Tree to Bottle
-
December 2021 Discussion About CTD by Lee Schwartzberg, MD, FACP, Professor of Medicine, University of Tennessee Health Science Center, and Napo Pharmaceuticals Scientific Advisory Board (SAB) Member.
-
Lisa Conte Interview with Sandra M. Swain, MD, FACP, FASCO (member of Jaguar/Napo Scientific Advisory Board).
-
Lisa Conte Interview with Dr. Kelly Shanahan, a former clinician and a metastatic breast cancer patient who is now a full-time independent patient advocate and a member of the Jaguar/Napo Scientific Advisory Board.
Source: Company Documents
Global Market Growth Opportunities

Jaguar Health is pursuing a pipeline of crofelemer formulations that span from supportive care for overactive bowel – which includes symptoms such as chronic debilitating diarrhea, GI urgency, and GI incontinence – to potential disease-modifying products in areas of high unmet need. The company’s initiatives represent an expansion into higher-value markets with greater potential to impact patient outcomes and lower the cost of care. The global prescription and OTC gastrointestinal agent market alone is projected to reach $21 billion by 2025, presenting sizable commercial opportunities if Jaguar can successfully develop and differentiate its products.

Source: Company Documents
In HIV-related diarrhea, Mytesi is the only FDA-approved treatment. About 70% of HIV/AIDS patients in the US are now over 50 and have lived with the virus for over ten years, often causing chronic gut complications that require symptomatic relief.
For cancer therapy-related diarrhea (CTD), 1.9 million new cancer cases were projected in the US for 2023. Up to 80% of chemotherapy patients experience diarrhea, requiring treatment delays/changes. By preventing CTD, crofelemer could support better adherence and outcomes. The global market for a comparable supportive care indication, chemotherapy-induced nausea and vomiting (CINV), is projected by market research firm iHealthcareAnalyst to reach a value of $3.9 billion by 2029
In short bowel syndrome (SBS) and congenital diarrheal disorders like microvillus inclusion disease (MVID), crofelemer may reduce dependence on parenteral nutrition. About 10,000-20,000 people in the US have SBS. MVID is an ultra-rare disease affecting a couple of hundred patients globally. The global SBS market alone is projected to grow to $4.6 billion by 2027, according to market research firm Vision Research Reports.
For diarrhea-predominant IBS, which impacts an estimated 15% of US adults, crofelemer would target the root cause of chronic watery diarrhea, unlike current treatments. Competitors have estimated peak US sales above $1 billion annually for drugs in this category. Effective relief of IBS-D symptoms remains a major unmet need.
Finally, in infectious diarrhea from pathogens like Vibrio cholerae, the company’s NP-300 drug product candidate may qualify for a Priority Review Voucher (PRV) from the FDA. PRVs are transferable and, in past transactions by other companies, have sold for values ranging from $67 million to $350 million. Additionally, it may meet a global need for affordable infection control in developing markets. In September 2023, the FDA activated the company’s Investigational New Drug (IND) application for NP-300 for crofelemer for this indication.
In summary, Jaguar Health's crofelemer pipeline spans high-value offerings in potential multi-billion-dollar markets where it can fill significant unmet needs related to diarrhea control and intestinal fluid regulation if successfully developed and commercialized.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
2023 Critical Activities to Unlock Jaguar Health's Value Potential

Jaguar Health has laid out several important goals and targets for 2023 through 2024:
A major milestone will be the expected availability in late October 2023 of the primary endpoint data for Napo’s pivotal Phase 3 OnTarget trial of crofelemer for preventative treatment of chemotherapy-induced overactive bowel (CIOB) in adults with cancer on targeted therapy. The primary endpoint looks at the study's main goal – evaluating crofelemer's effectiveness in reducing diarrhea rates. Jaguar Health anticipates being able to present additional data from this pivotal trial data at a medical conference in December 2023.
An independent pilot phase 2 study of crofelemer for the management of neratinib-associated diarrhea in patients with HER2+ early-stage breast cancer indicates that crofelemer, Napo’s FDA-approved drug, may be effective for the management of neratinib-induced diarrhea. The results of the study, which was designed by the study's principal investigator, Jo Chien, MD, and published in the peer-reviewed journal Clinical Breast Cancer, are consistent with the results of a preclinical dog study evaluating the effects of crofelemer in improving diarrhea associated with neratinib, a tyrosine kinase inhibitor (TKI), presented at the American Association for Cancer Research Virtual Annual Meeting II in June 2020.
Jaguar Health's pipeline includes pursuing crofelemer for short bowel syndrome (SBS) and congenital diarrheal disorders (CDD), which both qualify as rare diseases.
Source: Company Documents
Jaguar Health is supporting proof-of-concept clinical trials of crofelemer conducted by independent investigators for SBS and CDD. Proof-of-concept trials can provide early evidence that a drug may be effective. Jaguar Health expects that results from SBS and CDD proof-of-concept studies will be available before the end of 2023 in the first half of 2024.
The company believes the SBS and CDD proof-of-concept data could support expanded patient access to crofelemer through early access programs in specific European countries starting as early as 2024. Early access programs allow patients to receive a medicine before it is fully approved and can generate revenue while also allowing faster access for patients with serious diseases.
Also, in the second half of 2023, Jaguar Health expects the results of a canine study they completed evaluating crofelemer for preventing chemotherapy-related diarrhea in dogs to be published. This canine data could further support crofelemer's use for managing diarrhea from cancer treatments in humans.
Jaguar Health is targeting business development partnerships related to its pipeline and global commercialization efforts in 2023. Partnerships with other companies can help extend crofelemer's reach by licensing rights in certain geographies and disease areas.
Jaguar Health’s major clinical trial readouts, publications, and regulatory milestones spanning multiple programs slated for 2023-2024 all bode well for the company. Meeting these ambitious goals could significantly advance crofelemer toward approvals in cancer therapy-related diarrhea, SBS, and CDD while also expanding real-world evidence and patient access. Jaguar Health aims to execute new partnerships to extend commercial reach globally. Delivering on these key milestones will be critical for Jaguar Health's value and future growth potential.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Important Safety Information About Canalevia - For oral use in dogs only. Not for use in humans. Keep Canalevia-CA1 (crofelemer delayed-release tablets) in a secure location out of reach of children and other animals. Consult a physician in case of accidental ingestion by humans. Do not use in dogs that have a known hypersensitivity to crofelemer. Prior to using Canalevia-CA1, rule out infectious etiologies of diarrhea. Canalevia-CA1 is a conditionally approved drug indicated for the treatment of chemotherapy-induced diarrhea in dogs. The most common adverse reactions included decreased appetite, decreased activity, dehydration, abdominal pain, and vomiting. Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed. It is a violation of Federal law to use this product other than as directed in the labeling. Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-552. See full Prescribing Information at Canalevia.com.
Commercial Rights and Partnerships

Source: Company Documents
Jaguar Health holds global commercial rights to Mytesi® (crofelemer), its FDA-approved drug for the symptomatic relief of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. Jaguar also holds global rights to developing and commercializing crofelemer for multiple possible follow-on indications, including cancer therapy-related diarrhea.
In the animal health area, Jaguar holds the global rights to Canalevia®-CA1, its oral prescription drug FDA conditionally approved for the treatment of chemotherapy-induced diarrhea (CID) in dogs. Jaguar is focused on commercializing Canalevia-CA1 for CID in dogs in the United States.
In Europe, Jaguar has licensed the rights to crofelemer for short bowel syndrome, congenital diarrheal disorders, and HIV-related diarrhea to Jaguar family company Napo Therapeutics. This initiative provides Napo Therapeutics the exclusive rights to develop and commercialize crofelemer in Europe for these indications.
Additionally, Jaguar has partnered with Quadri Pharma for the potential commercialization of Mytesi in select countries in the Middle East and North Africa region. Quadri Pharma has exclusive promotional, distribution, and commercialization rights for Mytesi in these territories, following any necessary regulatory approvals.
Jaguar also has an agreement with Knight Therapeutics for the potential commercialization of Mytesi and other Jaguar prescription products in Canada and Israel. Knight holds exclusive distribution and commercialization rights in these territories.
Overall, Jaguar retains global rights to Mytesi, Canalevia-CA1, and the crofelemer pipeline while also retaining the ability to execute select partnerships to extend commercialization in key international markets. The company’s partnerships give Jaguar control over its core assets while leveraging partners to maximize the global value of its products.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Important Safety Information About Canalevia - For oral use in dogs only. Not for use in humans. Keep Canalevia-CA1 (crofelemer delayed-release tablets) in a secure location out of reach of children and other animals. Consult a physician in case of accidental ingestion by humans. Do not use in dogs that have a known hypersensitivity to crofelemer. Prior to using Canalevia-CA1, rule out infectious etiologies of diarrhea. Canalevia-CA1 is a conditionally approved drug indicated for the treatment of chemotherapy-induced diarrhea in dogs. The most common adverse reactions included decreased appetite, decreased activity, dehydration, abdominal pain, and vomiting. Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed. It is a violation of Federal law to use this product other than as directed in the labeling. Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-552. See full Prescribing Information at Canalevia.com.
Jaguar and Magdalena Biosciences Collaboration

Source: Company Documents
Beyond its core focus, Jaguar Health has invested in an intriguing new venture targeting mental health disorders through natural plant-based prescription drugs. This initiative, known as the Entheogen Therapeutics Initiative (ETI), exemplifies Jaguar Health's long-term vision to leverage its extensive knowledge of ethnobotanical plant medicine to develop innovative therapies across various disease areas.
The ETI is centered around exploring plants with potential mental health benefits, with an initial focus on discovering treatments for depression. This novel program aligns with Jaguar Health's mission to sustainably develop first-in-class medicines from rainforest plants through collaborations with indigenous communities. Jaguar Health's recent formation of the joint venture Magdalena Biosciences crystallizes the ETI strategy into a concrete drug development vehicle targeting major mental health needs.
Origins of Jaguar Health's Mental Health Focus
Jaguar Health's interest in expanding into mental health traces back to the experience of its management team members at the company's predecessor, Shaman Pharmaceuticals. In the 1990s, Shaman conducted extensive global ethnobotanical research, yielding a library of over 2,300 medicinal plants used in traditional healing systems. The team's field studies in Nigeria investigated Native African plants like Picralima nitida, which traditional healers have used to treat psychotic disorders.
When Jaguar Health acquired this plant library from the bankruptcy of Shaman in 2001, it gained a unique asset bridging indigenous medicine and Western pharmaceutical science. Jaguar Health has continued to build upon this library through additional rainforest research. Hence, Jaguar Health possesses unparalleled knowledge of medicinal plants, including many with potential but unexplored effects on the mind and mental health.
The Entheogen Therapeutics Initiative
Building upon this foundation, in 2021, Jaguar Health launched the Entheogen Therapeutics Initiative to formally pursue drug candidates from psychoactive plants for various mental health indications. The term "entheogen" refers to plants that induce spiritual or mystical experiences, many of which have traditional ceremonial uses. Jaguar Health's ETI goes beyond exploring recreational uses, focusing instead on developing rigorously tested prescription drugs for specific medical needs.
The ETI is advised by a Scientific Strategy Team of leading ethnobotanists, physicians, and pharmacologists, including several experts involved in the original Shaman library of plants. This team's knowledge of plant-based medicine and connections with indigenous communities is invaluable for identifying promising new drug candidates. Jaguar Health also chairs an ETI Ethics Board to ensure activities adhere to ethical standards regarding traditional knowledge and fair benefit-sharing.
Initially, the ETI is targeting the discovery of plant-based treatments for mood disorders like depression, anxiety, and PTSD. Depression, in particular, represents a significant unmet medical need, with over 21 million Americans affected each year. Existing antidepressant drugs like SSRIs have limitations in efficacy and side effects, creating demand for new therapeutic approaches. Plants offer a rich starting point for psychiatric drug discovery, given their long use in traditional healing systems.
The ETI has already pinpointed lead compounds from Jaguar Health's library for further investigation. One example is alstonine, a compound derived from the Picralima nitida plant used by traditional healers in Nigeria to treat psychiatric conditions. While still early stage, alstonine could provide a novel treatment mechanism compared to existing antidepressant drugs.
Formation of Magdalena Biosciences Joint Venture
To accelerate ETI's long-term mission, Jaguar Health recently established an exciting joint venture called Magdalena Biosciences, focused specifically on developing plant-based drugs for mental health disorders. Formed in January 2023 in partnership with the Canadian company Filament Health and with backing from One Small Planet, Magdalena Biosciences represents a milestone in Jaguar Health's bold push into mental health.
Magdalena Biosciences is leveraging Jaguar Health's extensive plant library along with Filament Health's drug development expertise. The joint venture has an exclusive license to evaluate plants and extracts from Jaguar Health's collection for specified mental health indications.
The first goal is to identify and optimize plants that may have promise for treating adult ADHD. ADHD is a common disorder affecting over 10 million adults in the U.S., yet existing stimulant treatments have misuse potential. Non-stimulant drugs are limited, creating an opportunity for plant-based alternative prescription drugs with new mechanisms of action and improved safety profiles.
In the future, Magdalena Biosciences plans to pursue additional mood disorders like anxiety, depression, and PTSD. The ultimate vision is to partner with pharmaceutical companies to fully develop and commercialize the venture's botanical drug candidates.
Jaguar Health owns approximately 40% of Magdalena Biosciences, initially valued at $5 million based on One Small Planet's seed investment of $1 million. This ownership stake provides Jaguar Health equity participation in Magdalena Biosciences' long-term success. Jaguar Health also benefits near-term from the $1 million infusion into its plant library and ethnobotanical expertise.
Strategic Context and Implications
Jaguar Health's pursuit of plant-based mental health drugs represents a bold long-term diversification, leveraging the company's unparalleled botanical knowledge and a large library of plants with potential medicinal benefits into new disease areas beyond core gastrointestinal indications. The ETI and Magdalena Biosciences joint venture strategically expands Jaguar Health's pipeline, targeting major conditions like mood disorders and ADHD, which affect millions of patients globally.
The ETI focuses on natural plant compounds that differentiate Jaguar Health's drug candidates from conventional synthetic psychiatric medications. These new drugs may offer improved efficacy, safety, and abuse potential profiles that drive adoption. Additionally, the basis of traditional plant medicine aligns with growing consumer interest in natural remedies over conventional medicines.
Of course, Jaguar Health faces formidable obstacles in proving the safety and efficacy of complex botanical drugs for mental illness. Clinical development timelines are lengthy, costs substantial, and failure rates high. But Jaguar Health is taking a prudent long-term approach through the ETI and Magdalena Biosciences, leveraging its unparalleled experience in ethnobotanical drug discovery.
If ultimately successful, Jaguar Health's novel plant-based therapies could transform the treatment of depression, ADHD, and other psychiatric conditions. This effort would enable Jaguar Health to tap into a multibillion-dollar market and drive significant shareholder value. In the nearer term, the ETI and the Magdalena Biosciences joint venture expand Jaguar Health's portfolio pipeline beyond gastrointestinal medicine, mitigating risk through therapeutic diversification.
Overall, the promise of plant-based mental health prescription drugs makes this an intriguing, if still highly speculative, new frontier for Jaguar Health, warranting close monitoring in the years ahead.
Financial Summary

Jaguar Health, Inc. (NASDAQ: JAGX) is a commercial-stage pharmaceuticals company focused on developing novel proprietary prescription medicines sustainably derived from plants from rainforest areas for people and animals with GI distress, specifically overactive bowel, which includes symptoms such as chronic debilitating diarrhea, GI urgency, and GI incontinence.
In 2022, Jaguar reported total revenues of $12 million, a significant 175.8% increase compared to revenues of $4.3 million in 2021. The revenue growth was primarily driven by increased sales of their human drug Mytesi® for symptomatic relief of noninfectious diarrhea in HIV/AIDS patients. This growth reflects the company's transition from a wholesaler distribution model to distribution through specialty pharmacies, resulting in lower discounts, rebates, and fees than the prior year. Jaguar also launched sales of their new animal drug Canalevia®-CA1 for chemotherapy-induced diarrhea in dogs in 2022, generating $167,000 in initial revenues.

Source: Company Documents
Despite strong revenue growth, Jaguar continued to report net losses in 2022 of $48.4 million, although this was an 8% improvement from net losses of $52.6 million in 2021. The losses are attributed to high operating expenses related to R&D for Jaguar's pipeline programs, including crofelemer for cancer therapy-related diarrhea and commercialization activities for Mytesi and Canalevia-CA1. Key expense items in 2022 included R&D expenses of $17.6 million, sales and marketing expenses of $8.8 million, and G&A expenses of $17.9 million.
Jaguar is progressing with developing crofelemer for additional indications beyond HIV-related diarrhea, including chemotherapy-induced overactive bowel (CIOB) and inflammatory bowel diseases. Crofelemer is the subject of the OnTarget study, Napo’s ongoing pivotal Phase 3 clinical trial for preventative treatment of CIOB in adults with cancer. Top-line data for the primary endpoint from this trial is expected at the end of October 2023.
The company has received orphan drug designations from the FDA and EMA for crofelemer for two rare diseases: short bowel syndrome (SBS) and microvillus inclusion disease (MVID, a congenital diarrheal disorder (CDD). Jaguar is supporting third-party investigational proof-of-concept studies of crofelemer for these indications that could enable early patient access in Europe, possibly in 2024. Further pipeline programs include NP-300, a second-generation anti-secretory agent for the treatment of moderate-to-severe diarrhea from bacterial, viral, and parasitic infections, including Vibrio cholerae, the bacterium that causes cholera. In September 2023, the FDA activated the company’s Investigational New Drug (IND) application for NP-300 for crofelemer for this indication. NP-300 has a similar mechanism of action as crofelemer but is less costly to produce. Jaguar also formed a joint venture in 2023 with Filament Health, called Magdalena Biosciences, to develop novel plant-based drugs for mental health indications, including ADHD, depression, and anxiety.
In animal health, Jaguar now markets Canalevia-CA1 for the treatment of chemotherapy-induced diarrhea in dogs under FDA conditional approval.
For long-term investors, Jaguar Health represents an early-stage biopharma with high-risk but potentially high reward as it aims to establish a differentiated pipeline of first-in-class plant-based medicines.
About Mytesi - Mytesi (crofelemer) is an antidiarrheal indicated for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy (ART). Mytesi is not indicated for the treatment of infectious diarrhea. Rule out infectious etiologies of diarrhea before starting Mytesi. If infectious etiologies are not considered, there is a risk that patients with infectious etiologies will not receive the appropriate therapy and their disease may worsen. In clinical studies, the most common adverse reactions occurring at a rate greater than placebo were upper respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%), flatulence (3.1%), and increased bilirubin (3.1%). See full Prescribing Information at Mytesi.com. Crofelemer, the active ingredient in Mytesi, is a botanical (plant-based) drug extracted and purified from the red bark sap of the medicinal Croton lechleri tree in the Amazon rainforest. Napo has established a sustainable harvesting program for crofelemer to ensure a high degree of quality and ecological integrity.
Important Safety Information About Canalevia - For oral use in dogs only. Not for use in humans. Keep Canalevia-CA1 (crofelemer delayed-release tablets) in a secure location out of reach of children and other animals. Consult a physician in case of accidental ingestion by humans. Do not use in dogs that have a known hypersensitivity to crofelemer. Prior to using Canalevia-CA1, rule out infectious etiologies of diarrhea. Canalevia-CA1 is a conditionally approved drug indicated for the treatment of chemotherapy-induced diarrhea in dogs. The most common adverse reactions included decreased appetite, decreased activity, dehydration, abdominal pain, and vomiting. Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed. It is a violation of Federal law to use this product other than as directed in the labeling. Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-552. See full Prescribing Information at Canalevia.com.
Analyst Coverage

Jaguar Health is followed by several seasoned biopharma analysts at leading investment banks and research boutiques. This sell-side coverage provides important visibility and insights on Jaguar Health to the institutional investment community.

Source: Company Documents
A Deep and Seasoned Leadership Team

Jaguar Health has assembled an experienced leadership team with decades of combined expertise across pharmaceutical R&D, clinical development, manufacturing, business development, commercialization, legal, and financial operations.
The management team is led by Lisa Conte, the company's founder, president, and CEO, who has over 30 years of experience pioneering plant-based prescription medicines. Other key executives include Pravin Chaturvedi, Ph.D., the company’s Chief Scientific Officer and Chair of the Scientific Advisory Board, and Chief Medical Officer Dr. Darlene Horton, who has over 25 years of experience overseeing clinical development in the biopharma industry. This seasoned leadership team provides Jaguar Health with the strategic vision and operational capabilities to advance the company's pipeline of novel plant-based therapies.
Source: Company Documents
Lisa Conte is the founder, president, and chief executive officer, and a member of the board of directors of Jaguar Health, a commercial-stage pharmaceuticals company committed to discovering, developing, and commercializing plant-based prescription medicines for urgent global health needs. In July 2017, two companies founded by Ms. Conte—Napo Pharmaceuticals, a human-focused pharmaceuticals company, and Jaguar Animal Health, the veterinary-focused licensor of all of Napo’s technology—merged and now comprise Jaguar Health. In 1989, Ms. Conte founded Shaman Pharmaceutical and pioneered plant-based prescription medicine investigation and development for more than 30 years. Ms. Conte is a member of the board of directors of Healing Forest Conservatory, serves on the Editorial Advisory Board of Life Science Leader magazine, and serves on the Leadership Council of Pure Earth. She holds an MS in Physiology and Pharmacology from the University of California, San Diego, and an MBA and AB in Biochemistry from Dartmouth College.
Steven King, Ph.D., has served as Chief of Sustainable Supply, Ethnobotanical Research, and Intellectual Property since 2020. He joined the company in 2002 as Senior Vice President of Sustainable Supply, Ethnobotanical Research, and IP. Before that, he was Vice President of Ethnobotany and Conservation at Shaman Pharmaceuticals. He has a Ph.D. in Biology from the Institute of Economic Botany at the New York Botanical Garden and City University of New York and an MS in Biology from the same institutions.
Pravin Chaturvedi, Ph.D., has been Chief Scientific Officer since 2022 and serves as Chair of the Scientific Advisory Board. He has over 30 years of experience in the pharmaceutical industry and has participated in developing multiple approved drugs. He was previously President and CSO of Napo from 2006-2013. He co-founded several biotech companies, including Scion Pharmaceuticals, IndUS Pharmaceuticals, and Oceanyx Pharmaceuticals. He has a Ph.D. in Pharmaceutical Sciences from West Virginia University and a Bachelor's in Pharmacy from the University of Bombay.
Carol R. Lizak has been Chief Financial Officer since 2021. She joined the company in 2019 as Vice President of Finance and Corporate Controller and was promoted to Chief Accounting Officer and then Senior Vice President of Finance before becoming CFO. She has over 20 years of finance experience, including roles at Zosano Pharma, Quantum Secure, Alexza Pharmaceuticals, and HID Global. She holds an MBA from Pepperdine University Graziadio School of Business and Management and a BS in Business Administration from the University of Santo Tomas.
Karen J. Brunke, Ph.D., brings to Jaguar over 30 years of scientific, operational, clinical, senior executive, and corporate development experience in both large and small biotechnology companies. Dr. Brunke has been primarily responsible for negotiating multiple partnerships and licenses in business and corporate development. Dr. Brunke received her BA degree in Biochemistry and a Ph.D. in Microbiology from the University of Pennsylvania.
Darlene Horton, MD, brings over 25 years of clinical research and development, medical affairs, senior executive, and consulting experience in developing investigational and commercialized biopharmaceutical and drug-device combination products. She has extensive clinical development experience in multiple therapeutic areas. Before joining Napo Pharmaceuticals, she led clinical development and regulatory strategy as CMO at Coherus Biosciences, Itero Biopharmaceuticals, and SMC Biotechnology. Dr. Horton completed her Pediatric Cardiology fellowship and Pediatrics Residency at UCSF. She earned an MD and BS in Microbiology from the University of Florida.
David Sesin, Ph.D., is a pharmaceutical scientist with more than 30 years of experience, from drug discovery to manufacturing. Before serving as Jaguar’s CMO, he was Director of Chemistry and QHSE at Bayer CropScience and Director of Chemistry at AgraQuest, Inc. he spent nine years with Shaman Pharmaceuticals. Dr. Sesin holds a Ph.D. from the University of Utah.
Jonathan S. Wolin, JD, has been Chief of Staff and General Counsel since 2019. Before being promoted, he joined the company in 2018 as Chief Compliance Officer and Corporate Counsel. Previously he was an independent consultant, Chief Administrative Officer at Braden Partners, and Chief Compliance Officer at Natera and Braden Partners, among other roles. He holds a JD from The Catholic University of America Columbus School of Law, an MBA from The George Washington University School of Business, and a BS in Accounting from the University of Maryland
Ian Wendt has been Chief Commercial Officer since 2020 after joining as VP of Commercial Strategy in 2019. He has over 15 years of experience in pharmaceutical and biotech sales, marketing, and operations at companies including Gilead Sciences, Boehringer Ingelheim, and Roxane Laboratories. He has an MBA from Dalhousie University in Nova Scotia and a BSc from Acadia University.
SEC Filings
Financials
Risks & Disclosures

This communication is neither an offer to sell nor a solicitation of an offer to buy, nor a recommendation of any securities of the company mentioned herein.
Newton Golf Company (the “Company”) has reviewed the content of this page as well as the accompanying presentation (“Company Presentation”) displayed on this page. To the best of its knowledge, the Company does not believe this content to be misleading or inaccurate in any material respect, nor does it believe there are any material omissions with respect to such content. The Company does not believe the contents of the page or the Company Presentation to contain any non-public material information.
Information and opinions presented in the Company Presentation are provided by the Company, and b2iDigital makes no representation as to their accuracy or completeness. The information contained on this page is not intended to constitute any form of advice, and the information provided is not intended to provide a sufficient basis on which to make an investment decision. It is not investment research, nor does it constitute a research recommendation, as it does not constitute substantive research or analysis. This information is not to be relied upon in substitution for the exercise of independent judgment.
Information, opinions and estimates contained on this page or in the Company Presentation reflect judgments by the Company as of the original date of publication by the Company and are subject to change without notice. Past performance should not be taken as an indication or guarantee of future performance, and no representation or warranty, express or implied is made regarding future performance.
A complete description of the risks and uncertainties relating to the Company and its securities can be found in the company's filings with the U.S. Securities and Exchange Commission available for free at www.sec.gov.
Information on this page may relate to penny stocks, which may also be referred to as low-priced stocks. Penny stocks are low-priced shares typically issued by small companies. Penny stocks involve greater than normal risk, they may be less liquid than other stocks (i.e., more difficult to sell), and there may be less reliable information available regarding such stocks. Investors in penny stocks should be prepared for the possibility that they may lose their entire investment.
b2i digital or its related entities may own securities of the Company.
The Company is a client of b2i Digital. The Company agreed to pay b2i Digital no greater than $100,000 in cash for 12 months of digital marketing consulting and investor awareness services.
This communication includes forward-looking statements that involve risks, uncertainties and assumptions that are difficult to predict. Words and expressions reflecting optimism, satisfaction or disappointment with current prospects, as well as words such as “believes,” “hopes,” “intends,” “estimates,” “expects,” “projects,” “plans,” “anticipates” and variations thereof, or the use of future tense, identify forward-looking statements, but their absence does not mean that a statement is not forward-looking. The Company’s forward-looking statements are not guarantees of performance, and actual results could vary materially from those contained in or expressed by such statements due to risks, uncertainties and other factors. The Company urges readers to consider specifically the various risk factors identified in its most recent Form 10-K, and any risk factors or cautionary statements included in any subsequent Form 10-Q or Form 8-K, filed with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this communion. Except as required by law, the Company does not undertake any responsibility to update any forward-looking statements to take into account events or circumstances that occur after the date of this communication.
The Newton Golf Company management and investor relations team are available to talk to current and potential investors. They're happy to answer your questions and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
• Directly hear the Newton Golf Company Management story
• Ask your questions
• Submit the form below, and someone will get in touch with you as soon as possible
Note: Company management or its representative can only discuss and disclose information that is already available in the public domain. They will do their best to clarify such information to the extent permitted by securities law and industry regulations.








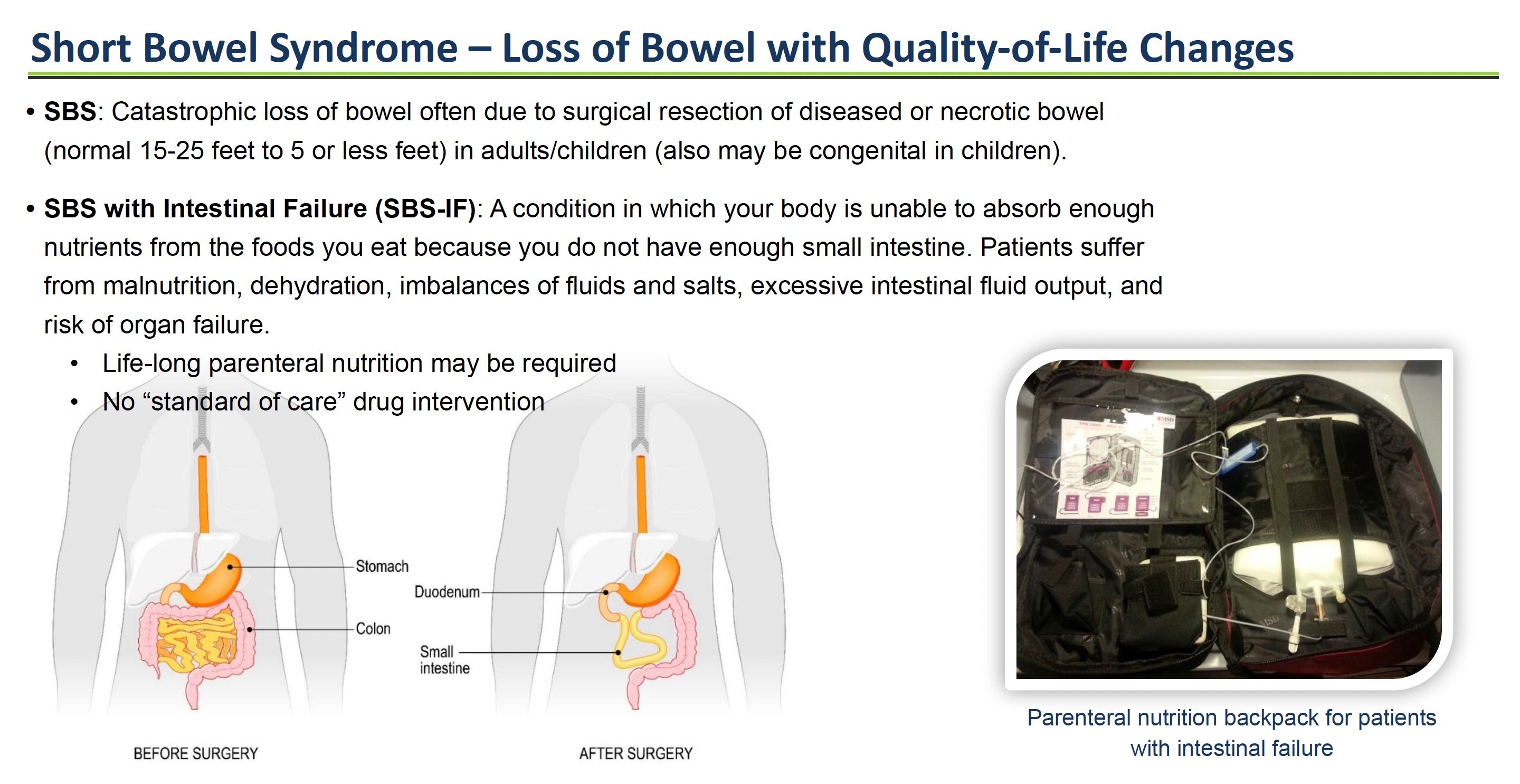

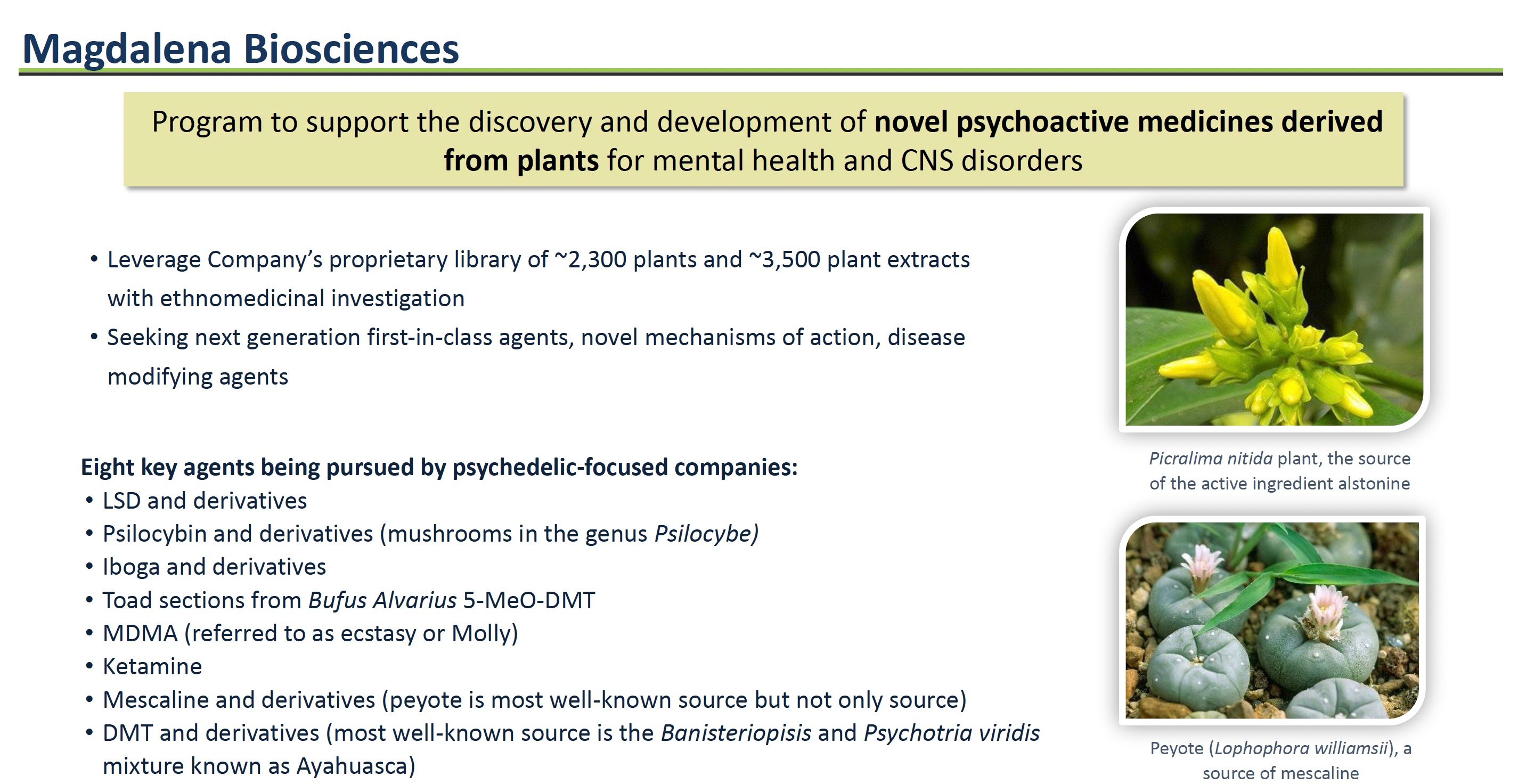





.png?width=194&height=122&name=NEWTON%20GOLF%20-%20LOGO%20-%20FINAL%20-%20V1%20-%20UPDATE%20-%20WHITE%20(1).png)

Premium Technology Focus
Physics-based innovations include patented Ultra-Low Balance Point putter technology and elongated bend profile shaft design.