BioCorRx, Inc
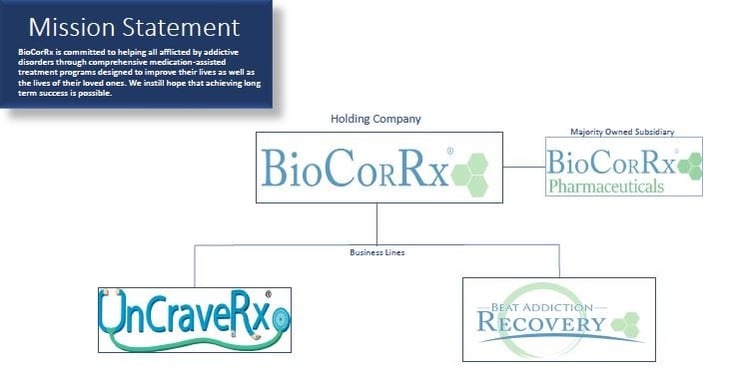
BioCorRx® is a leading-edge healthcare solutions company focused on improving the lives of those struggling with alcoholism, opioid abuse, as well as other addictive disorders.
The company's business lines include two operational programs, Beat Addiction Recovery, a Medication-Assisted Treatment program, and UnCraveRx®, a weight management program, in addition to an R&D subsidiary, BioCorRx Pharmaceuticals, Inc. Their pharmaceutical treatments utilize naltrexone, an FDA-approved drug that binds to receptors and prevents the release of dopamine.
OTCQB: BICX
IR Website: https://ir.biocorrx.com/
Headquarters: Anaheim, California
TALK TO MANAGEMENT
The BioCorRx management team is always available to talk to current and potential investors. They're happy to answer any questions you may have and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
Summary

BioCorRx® was founded to help people rebuild their lives by overcoming addiction. We believe that with the right support, people can live productive, fulfilling lives. Our leadership is comprised of doctors, forward-thinking experts, as well as some who have experienced the vicious cycle of addiction firsthand. We are committed to transforming lives and making the world a better place for us all.
The Cost of Addiction
Addiction is a powerful disorder that affects over 23 million people in the U.S. alone1. Addiction costs an estimated $700 billion each year2.
Addiction in America3
-
Over 85% of people with alcohol abuse or dependence go untreated
-
Over 2 million people in the U.S. abuse prescription pain relievers
-
An estimated 900,000 people use Heroin (an illegal opiate)
- National Survey on Drug Use and Health by the Substance Abuse and Mental Health Services Administration (SAMHSA)
- See American Society of Addiction Medicine (ASAM) Public Policy Statement on Treatment for Alcohol and Other Drug Addiction, Adopted: May 01, 1980, Revised: January 01, 2010
- National Survey on Drug Use and Health by the Substance Abuse and Mental Health Services Administration (SAMHSA)
The Addiction Epidemic
[*]. Substance Abuse and Mental Health Services Administration. (2018). Key Substance Use and Mental Health Indicators in the United States: Results from the 2017 National Survey on Drug Use and Health. |
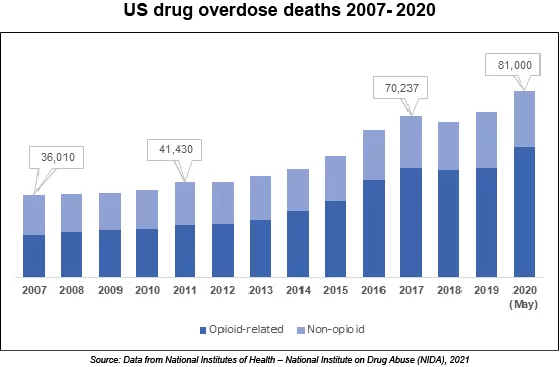
U.S. Drug Overdose Deaths Hits An Alarming Record During COVID-19 Pandemic. More than 83,000 drug overdose deaths occurred during the 12 months ending in May 2020, the most ever recorded during a one year period, according to the Centers for Disease Control and Prevention. |
What Makes the BioCorRx® Recovery Program Different?
If the treating physician determines to utilize a medication-assisted treatment (MAT) program, a multi-pronged approach can assist in addressing the underlying physical and behavioral issues of alcoholism and opioid addiction.
Our proprietary, modular CBT program can be tailored to help those suffering from substance use disorders, and is designed to help prepare them for a life without their desired substance.
This multi-tiered approach may result in lower patient drop-out possibly due to reduced cravings if the treating physician uses an anti-craving medication. The program can be done very discreetly on an outpatient basis with virtually no major disruptions from daily life.
Press Releases
Investor Presentation


To download the BioCorRx investor presentation, please fill out the form below.
Stock Chart (Intraday)
Stock Chart (Historical)
CEO Interview: Lourdes Felix Provides Update

BioCorRx Provides Update on R&D Candidates, Mental Health Awareness and Clinical Development
Apr 14, 2022
SNNLive spoke with Lourdes Felix, CEO and CFO of BioCorRx Inc. (OTCQB: BICX)
0:57 Quick overview of BioCorRx
1:55 Update in the last 12 months: R&D product candidate - BICX-104 - preclinical studies, getting ready to start clinical studies for BICX-104
3:30 Mental Health crisis and awareness - tailwind?
4:53 Value catalysts for the rest of 2022
Market Opportunity

Addiction by the Numbers
-
38 million Americans are heavy drinkers, according to the CDC
-
Per the CDC, more than 12% of the population are alcohol dependent
-
23.5 million American adults are addicted to drugs and or alcohol
-
100 Americans die from preventable overdose every day, per the CDC
-
770% increase in those seeking treatment for opioid addiction in the last 15 years
-
In 2010, more than 12 million people reported using pain killers non-medically
-
85% of the people with alcohol abuse or dependence go untreated
-
Heroin is the most widely used opiate, with more than 900,000 estimated users

Weight Loss Market
Addressing Unmet Challenges in Addiction Treatment
BioCorRx has created the BioCorRx® Recovery Program to address some of the biggest, previously unmet challenges related to addiction treatment: the low rates of long-term sobriety. One study, for example, found that only 46% of addicts who attended a residential treatment program managed to stay in recovery (only about 40% of that number managed to stay completely clean and sober).
An elegant, simple solution to reduce cravings
In the past, treatment has been focused on the emotional needs and behaviors associated with addiction while ignoring or underutilizing pharmacological solutions that go to the heart of addiction: the intense craving to use.
BioCorRx® is uniquely poised to gain significant market share of the $35 billion annual treatment market in the US.
Addiction Has Surged

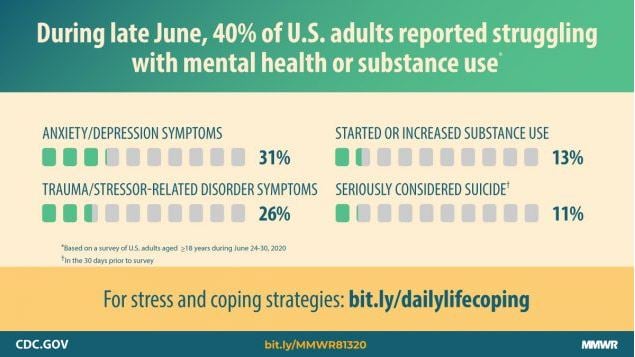
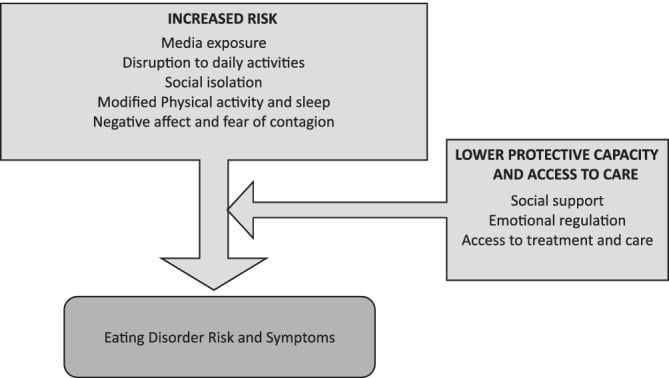
The COVID-19 pandemic has become a strong and unfortunate catalyst for increasing addiction issues. According to the CDC, 13% of Americans started or increased substance use from June 2019 to June 2020 as a way to cope with the impacts of stay-at-home and distancing policies.
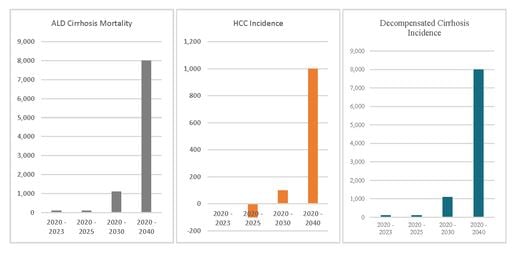
According to a peer reviewed survey from the International Journal of Environmental Research and Public Health, 60% of Americans reported drinking more alcohol during the course of the pandemic. A study by the Massachusetts General Hospital estimates that an additional 8,000 cirrhosis deaths will eventually be attributable to the effects of the pandemic.
Additionally, a Ball State College of Health survey reported that 39% of Americans admitted to overeating during the course of the pandemic.
BioCorRx provides solutions for all of these issues and is well positioned to thrive while helping better lives in the process.
BioCorRx Pharmaceuticals


BioCorRx Pharmaceuticals, Inc. focuses on the development and regulatory approval of medications utilizing 505(b)(2) pathway and/or in the addiction treatment industry. The lead candidate is a naltrexone pellet implanted subcutaneously being developed to address both opioid and alcohol use disorders.
They are seeking FDA approval of the naltrexone pellet and exploring other products for regulatory approval. They were awarded approximately $5.7 million from National Institute on Drug Abuse (NIDA) in January 2019 for development.
Preclinical GLP studies commenced March 2020. Investigational New Drug (IND) was cleared in May 2021 by the FDA and first-in-human clinical trials will be done at Orange County Research Center.

Naltrexone Pellet

Naltrexone Pellet - for the treatment of opioid and alcohol use disorders
The Naltrexone Pellet is a biodegradable extended-release subcutaneous pellet in development. It is expected to deliver therapeutic naltrexone plasma levels for approximately 3 months. A similar product has been licensed in Russia for several years under the name Prodetoxone™. Prodetoxone™ has been through multiple trials conducted at the St. Petersburg Scientific-Research Center of Addictions and Psychopharmacology, Pavlov Medical University, and in conjunction with the University of Pennsylvania, Department of Psychiatry, Philadelphia, USA.

The Naltrexone Pellet addresses both alcohol and opioid use disorders. It fully biodegrades, eliminating the need to remove and replace. It also contains non-addictive active pharmaceutical ingredients, all of which are potential advantages over other products used to treat addiction.
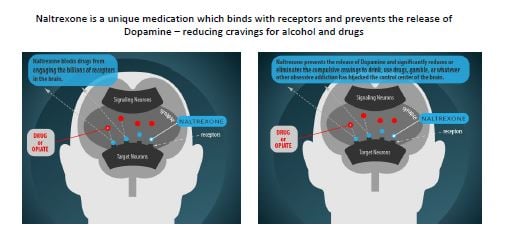
Additionally, it is expected to maintain therapeutic plasma levels for approximately 90 days, and expected to be easier to remove if required for any reason, giving it a potential advantage over the current injectable naltrexone product.
Meeting Physiological and Psychological Needs

Designed to address alcoholism and certain opioid addictions, the BioCorRx® Recovery Program is used by independent treatment centers and physicians in the United States. The program consists of BioCorRx’s proprietary cognitive behavioral therapy (CBT) program, peer recovery support mobile application and may include the use of certain medications typically used for the treatment of substance use disorder (SUD). Which medication used, if any, is at the sole discretion of the treating physician in consultation with their patient. The most common medication used in the program is naltrexone in various forms (oral, injectable, implantable pellet).
If the treating physician determines to utilize a medication-assisted treatment (MAT) program, a multi-pronged approach can assist in addressing the underlying physical and behavioral issues of alcohol and opioid addiction.

Our proprietary, modular CBT program can be tailored to help those suffering from substance use disorders, and is designed to help prepare them for a life without their desired substance.
This multi-tiered approach may result in lower patient drop-out possibly due to reduced cravings if the treating physician uses an anti-craving medication. The program can be done very discreetly on an outpatient basis with virtually no major disruptions from daily life, with mobile support designed to fit into the patient's everyday life.
Beat Addiction Recovery Mobile App
|
 |
 |
Tackling Obesity

It should not be a surprise to most that obesity is a very significant public health issues in America. According to a The Behavioral Risk Factor Surveillance System study in 2019 all states had incidences of obesity in over 20% of adults, and 35% or more of adults were considered obese in 12 states.
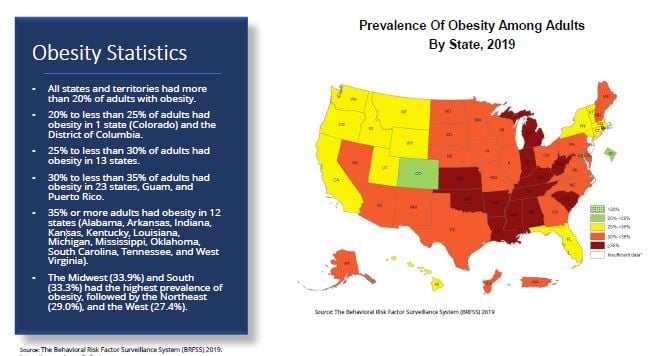
The public health costs as a result are staggering. According to the George Washington University, estimates to the medical cost of obesity in America leads to between $147 billion and $210 billion in medical spending per year, and is responsible for $61.8 billion in Medicare and Medicaid spending per year.
BioCorRx's solution for fighting obesity is multi-faceted and comprehensive. It includes medical aspects to both diagnose and treat obesity, virtual support to address patient needs, and group support to help the patient stay on track.
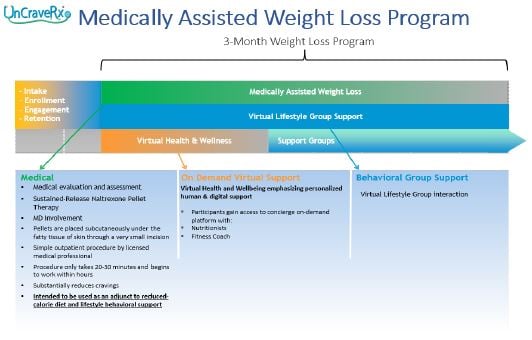
Objectives and Milestones


-
Seeking FDA approval of implantable naltrexone pellet
-
Formed a Scientific Advisory board, includes Dr. David Gastfriend, Dr George Woody, and Dr. Evgeny Krupitsky
-
Gastfriend previously served as VP of Scientific Communications for Alkermes; heavily involved with Vivitrol® and
-
Drs. Woody and Krupitsky are frequent principal investigators for naltrexone implants
-
Designed study with the help of the late Dr. Bal S. Brar over 25 years of experience for drug and device development as well as worldwide regulatory submission of 50 INDs/510K’s and 505(b)(2)’s; and approval of 8 NDA’s
-
Partnered with Innovative Science Solutions, LLC, a leading scientific consulting firm, to help guide the Company’s regulatory strategy for FDA submission
-
Expanded development and manufacturing relationship with Recro (formerly IriSys) to support BICX104
-
505(B)(2) pathway deemed acceptable by FDA
-
As a result of meeting, seeking dual indication for both alcohol and opioid use disorders
-
Preclinical and clinical studies for safety, pharmacokinetics, and human factors (not planning to do efficacy studies per FDA meeting)
-
-
Received National Institute on Drug Abuse (NIDA) grant of approximately $5.7 million
-
Received FDA Clearance of Investigational New Drug (IND) application for BICX104
-
Awarded approx. $3.5 million second phase of NIDA Grant for the first in human clinical trial of BICX104 for total of nearly $9.2 million in grant funding to date
Financials
SEC Filings
Management Overview

Lourdes Felix
CEO, CFO and Director
Lourdes Felix is a female Hispanic entrepreneur and corporate finance executive with 30 years of combined experience in capital markets, public accounting and in the private sector. She presently serves as Chief Executive Officer, Chief Financial Officer and Director of BioCorRx Inc. (OTCQB: BICX), a leader in addiction treatment solutions and related disorders. She has been with BioCorRx since October 2012. Lourdes is one of the founders and President of BioCorRx Pharmaceuticals Inc., a majority owned subsidiary of BioCorRx Inc. She has been instrumental in capital procurement, completing multi-million dollar equity financing and accomplished in structuring and negotiating transactions and favorable terms with investment banks. Along with other executives of the company, rebranded the Company and restructured and expanded the business model to position it for long term growth in the addiction treatment space and drug development. Extensive experience with clinic operations management.
Prior to joining BioCorRx her experience was in the private sector, public accounting including audit and public company experience. She has expertise in finance, accounting, budgeting and internal control principals including GAAP, SEC, and SOX Compliance. Thorough knowledge of federal and state regulations. Successfully managed and produced SEC regulatory filings. She has extensive experience in developing and managing financial operations. Lourdes has provided treasury and cash management functions. Excellent leader with a track record of documented contributions leading to improved financial performance, heightened productivity, and enhanced internal controls. Led corporate relationships with various major accounting firms and attorneys in preparing SEC filings and audited financial statements. Lourdes is very active in the Hispanic community and speaks fluent Spanish. Lourdes holds a Bachelor of Science degree in Accounting from University of Phoenix. She is an MBA candidate at D’Amore-McKim School of Business, Northeastern University.
Brady Granier
President and Director
During the 12 years prior to joining BioCorRx® in June of 2013, Brady Granier had been involved in sales management, media sales and business development. Mr. Granier was employed at Clear Channel Media & Entertainment ("CCME"), where he had served in several positions from Account Executive to Director of Business Development and Local Sales Manager. He has also served as the Healthcare Category Manager for the Los Angeles division of CCME, the largest media company in the United States. During his tenure at CCME and other media companies, he worked on marketing campaigns for local businesses and physicians, as well as for national brands such as Neutrogena, New Line Cinema, Paramount Pictures, Samsung, AT&T, Coke, Dr. Pepper, Hansen's, Honda, MGM, Universal Studios and more. He also managed endorsements on the radio for Ryan Seacrest. In 2006, He received the coveted Pinnacle Award from CCME for being the top sales executive in the Western region. While serving as Director of Business Development, Mr. Granier grew new business by 49% in his first year in that role.
Granier was born and raised in the heart of Cajun Country in Southeast Louisiana where he started working at the age of 11 to help support his single mother and younger brother. After graduating with honors from high school, he attended college at Nicholls State University in Thibodaux, LA. He earned his Bachelor of Science degree in Nursing in 1995 and was a member of Sigma Theta Tau Honor Society and Phi Kappa Theta. During his nursing career, he specialized in the critical care areas of ER/ICU/CCU and CICU. He also moonlighted as a home health nurse, critical care air transport nurse and TV studio set medic. In 1996, he moved to California as a travel nurse and spent most of his remaining years in healthcare as the charge nurse in the emergency room at White Memorial Hospital in downtown Los Angeles. Mr. Granier continues to reside in the Los Angeles area with his family. He has also been a volunteer with Big Brothers of America.
Tom Welch
VP of Operations
Welch was a founder and Director of Operations at TAK Management from 2012 until 2015. TAK Management was responsible for the streamlining of clinical and financial operations for Start Fresh Alcohol and Opioid Recovery Clinic Inc. His responsibilities included placement and training of key personnel, billing, coding, and collections and establishing and maintaining relationships with outside vendors and physicians. Mr. Welch led a team that completely reorganized clinic operations. Tom’s responsibilities included Implementation of policies and procedures as well as creation of standard practice protocols integrated in to multiple locations. Additionally, Tom Implemented traditional media and Social Marketing strategies. Tom was also successful in negotiating reimbursement with major private insurance corporations across the U.S.
Prior to his tenure with TAK Management, Mr. Welch had been involved in the identifying and placement of talented executive level candidates nationwide in healthcare since 1999. In addition to doing both temporary and permanent placement as an Executive Healthcare recruiter, he had been engaged in consulting in the long term care, acute care, and rehab care marketplace. Mr. Welch created Benchmark Consulting Group LLC in 2001 in Los Angles California and expanded into Dallas Texas in 2005. He has been actively involved in assisting in infrastructure creation including, front and back office management, billing and coding personnel, clinical management and executive teams. Tom was responsible for negotiating multiple partnership agreements and contracts with major medical corporations and both the Military and Veterans Administration.
Welch started his career in Healthcare in Omaha Nebraska where he supervised the medivan program while training for his emergency medical technician license with Eastern Ambulance.
Dr. George Fallieras
Medical Consultant
Dr. George N. Fallieras comes from a family of physicians. Dr. Fallieras grew up in Tampa, Florida, and graduated Phi Beta Kappa from the University of Florida. He obtained his M.D. from the University of Tennessee, and did his residency training in New Orleans at the Tulane Health Science Center/Charity Hospital. Dr. Fallieras is double board certified in both Internal Medicine and Pediatrics. He has extensive emergency room, hospital inpatient, ICU, inpatient and outpatient detoxification, and outpatient recovery experience. He has served as the Medical Director for multiple large Inpatient Hospitalist programs. He is passionate about international medicine and serves on twice yearly missions trips to rural Dominican Republic and Port-au-Prince, Haiti. Dr. Fallieras has served as expert commentary on multiple news, television, and radio outlets.
Dr. Fallieras has always taken a keen interest in addiction medicine, in particular alcohol and opiate abuse. He has provided care to countless alcoholic and addicted patients in the acute hospital setting. He has seen innumerable cases of suffering individuals literally drinking themselves to death. With dread, he has made those difficult calls to parents notifying them of their child’s overdose and death. He has witnessed the profound physical and emotional burden that alcohol and drug abuse inflicts upon the individual and their families. He has seen the disappointment and shame in the eyes of very good and determined individuals who desperately want to quit drinking but are held down by the firm grasp of addiction. Like most people, he has first-hand knowledge of family members and loved ones who have endured this common struggle.
As a medical director for BioCorRx, he has established ethical and effective protocols. He believes addiction is a chronic “brain disease” and requires comprehensive treatment including physical and laboratory evaluation, vitamin and nutrient repletion, nutritional support, safe and comfortable detoxification, medications to eliminate or reduce cravings, psychological attention, emotional regulation, behavioral modification to identify/address/avoid/extinguish triggers, and long-term attention and surveillance (group setting and support most effective).
Risks & Disclosures

This communication is neither an offer to sell nor a solicitation of an offer to buy, nor a recommendation of any securities of the company mentioned herein.
BioCorRx Inc. (the “Company”) and its counsel have reviewed the content of this page as well as the accompanying presentation (“Company Presentation”) displayed on this page. To the best of its knowledge, the Company does not believe this content to be misleading or inaccurate in any material respect, nor does it believe there are any material omissions with respect to such content. The Company does not believe the contents of the page or the Company Presentation to contain any non-public material information.
Information and opinions presented in the Company Presentation are provided by the Company, and b2i digital makes no representation as to their accuracy or completeness. The information contained on this page is not intended to constitute any form of advice, and the information provided is not intended to provide a sufficient basis on which to make an investment decision. It is not investment research, nor does it constitute a research recommendation, as it does not constitute substantive research or analysis. This information is not to be relied upon in substitution for the exercise of independent judgment.
Information, opinions and estimates contained on this page or in the Company Presentation reflect judgments by the Company as of the original date of publication by the Company and are subject to change without notice. Past performance should not be taken as an indication or guarantee of future performance, and no representation or warranty, express or implied is made regarding future performance.
A complete description of the risks and uncertainties relating to the Company and its securities can be found in the company's filings with the U.S. Securities and Exchange Commission available for free at www.sec.gov.
Information on this page may relate to penny stocks, which may also be referred to as low-priced stocks. Penny stocks are low-priced shares typically issued by small companies. Penny stocks involve greater than normal risk, they may be less liquid than other stocks (i.e., more difficult to sell), and there may be less reliable information available regarding such stocks. Investors in penny stocks should be prepared for the possibility that they may lose their entire investment.
b2i digital or its related entities may own securities of the Company.
To comply with Rule 17(b) of the Securities Act of 1933, as amended, b2i Digital must provide full disclosure of all compensation received for investor awareness services provided by the Company. The Company is a client of b2i Digital. The Company agreed to pay b2i Digital no greater than $100,000 in cash for 12 months of digital marketing consulting and investor awareness services.
The BioCorRx management and investor relations team is available to talk to current and potential investors. They're happy to answer your questions and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
• Directly hear the BioCorRx story
• Ask your questions
• Submit the form below and someone will get in touch with you as soon as possible
Note: Company management or its representative can only discuss and disclose information that is already available in the public domain. They will do their best to clarify such information to the extent permitted by securities law and industry regulations.