First Wave BioPharma, Inc.
First Wave BioPharma, Inc. is a clinical-stage biopharmaceutical company developing targeted, non-systemic therapies for gastrointestinal (GI) diseases. The Company is currently advancing a therapeutic development pipeline with multiple Phase 2 clinical-stage programs built around three proprietary technologies – capeserod, a selective 5-HT4 receptor partial agonist that First Wave will pursue for gastrointestinal (GI) indications; the biologic adrulipase, a recombinant lipase enzyme designed to enable the digestion of fats and other nutrients in cystic fibrosis and chronic pancreatitis patients with exocrine pancreatic insufficiency; and niclosamide, an oral small molecule with anti-inflammatory properties for patients with inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease.
The Company's lead product candidate is adrulipase. In January 2023, First Wave initiated a Phase 2b bridging study evaluating an improved enteric microgranule formulation of adrulipase in CF patients with EPI. While initial topline data from this study announced in July 2023 indicated the new formulation was safe and improved over prior versions, it likely did not meet the primary efficacy endpoint. Additional Phase 2b study data will be reported towards the end of 2023.
In September 2023, First Wave announced an agreement with Sanofi (NASDAQ: SNY) to license Capeserod, a selective 5-HT4 receptor partial agonist, which First Wave will repurpose and develop for gastrointestinal (GI) indications such as pediatric ulcerative colitis (an orphan disease) and gastroparesis.
Headquartered in Boca Raton, Florida, First Wave continues working to advance its pipeline of non-systemic GI therapies. For more information, visit www.firstwavebio.com.
Nasdaq: FWBI
IR Website: https://firstwavebio.com/investors/overview
Headquarters: Boca Raton, FL
TALK TO MANAGEMENT
The First Wave BioPharma management team is always available to talk to current and potential investors. They're happy to answer any questions you may have and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
Summary

First Wave BioPharma is a clinical stage biotechnology company currently focused on the development of targeted, non-systemic therapies for gastrointestinal diseases.
-
Robust IP portfolio covering method, formulation and use indications; key patents secure for 15-20 years
-
Pipeline of gut-targeted GI therapies address significant unmet medical needs in billion-dollar markets
Source: Company Reports
Targeted, Non-Systemic Therapies for Gastrointestinal Diseases
THE SCIENCE
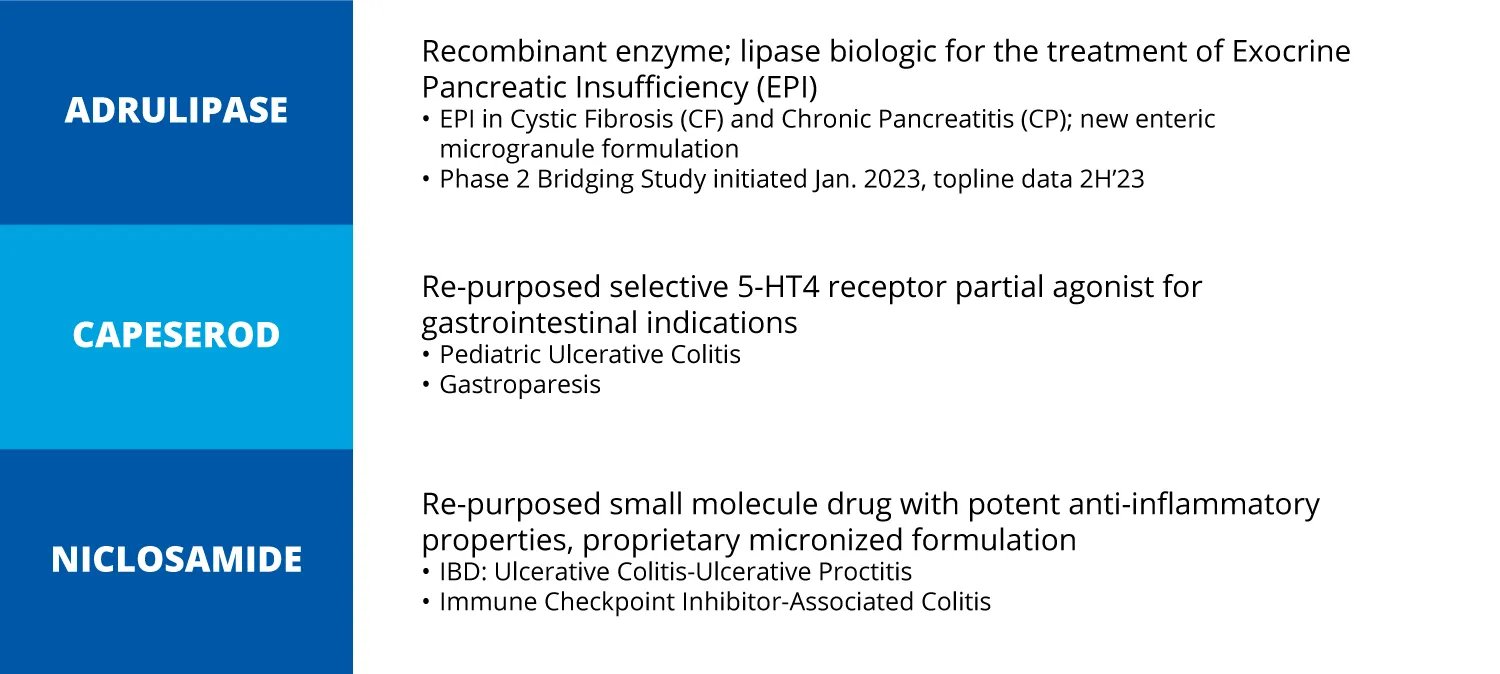
First Wave BioPharma is focused on developing targeted, non-systemic therapies for gastrointestinal (GI) diseases. The Company's current clinical programs center around its three proprietary technologies – adrulipase, capeserod, and niclosamide.
Source: Company Reports
THE PIPELINE
First Wave's lead product candidate is adrulipase, a recombinant lipase enzyme being developed as an oral, non-systemic biologic capsule for treating exocrine pancreatic insufficiency (EPI) in patients with cystic fibrosis and chronic pancreatitis.
In January 2023, the Company initiated a Phase 2b bridging study of an improved enteric microgranule formulation of adrulipase in CF patients with EPI. Initial topline data was reported in July 2023, and additional data will be reported towards the end of 2023.
In September 2023, First Wave announced an agreement with Sanofi (NASDAQ: SNY) to license Capeserod, a selective 5-HT4 receptor partial agonist, which First Wave will repurpose and develop for gastrointestinal (GI) indications such as pediatric ulcerative colitis (an orphan disease) and gastroparesis.
First Wave is also developing niclosamide formulations for inflammatory bowel diseases like ulcerative colitis and Crohn's disease. However, the development of the niclosamide programs is currently on hold due to financial constraints.
THE DEVELOPMENT STORY
First Wave continues efforts to advance its therapeutic pipeline of non-systemic GI therapies. The Company is focused on progressing the clinical development of adrulipase and exploring strategic options to move its niclosamide programs forward.
First Wave's goal is to help address the significant unmet medical need that exists for patients with debilitating gastrointestinal diseases. The Company is committed to protecting health and restoring quality of life by developing novel, targeted treatment options.
Press Releases
Investor Presentation


To download the First Wave BioPharma, Inc. investor presentation, please fill out the form below.
Stock Chart (Intraday)
Stock Chart (Historical)
Leadership In Commercialization

President and CEO James Sapirstein brings over 40 years of pharmaceutical experience to First Wave BioPharma, having held key positions at major companies, including Eli Lilly, Hoffman-LaRoche, Bristol Myers Squibb, and Gilead. He led the Global Marketing Team at Gilead and was instrumental in the market launch of their blockbuster HIV drugs, Viread and Truvada.
Mr. Sapirstein also co-founded Tobira Therapeutics, which was later acquired by Allergan for $1.7 billion, demonstrating his ability to create substantial value. With over 25 drug launches under his belt, he possesses the expertise needed to commercialize First Wave's therapies.
Additionally, First Wave has assembled an esteemed Scientific Advisory Board and Clinical Steering Committees. This exceptional scientific knowledge will be invaluable as the company advances its promising gastrointestinal pipeline focused on unmet medical needs.
The Gastroenterology Market

Gastroenterology is the branch of medicine that focuses on issues with the digestive system. First Wave BioPharma, Inc. is a clinical-stage biopharmaceutical company that develops targeted, non-systemic therapies for gastrointestinal (GI) diseases.
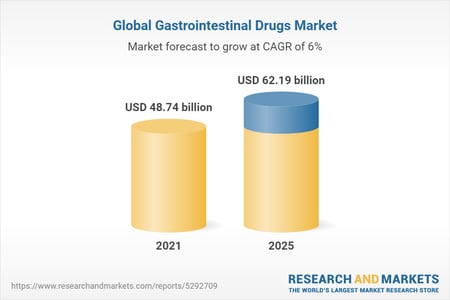
According to Research and Markets, the market for gastroenterologic therapeutics continues to grow, estimated to reach over $62 billion by 2025.
Source: researchandmarkets.com
First Wave is focused on treating inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease. The IBD treatment market is projected to reach $29.2 billion by 2027 with an 8.1% CAGR, according to Research and Markets.
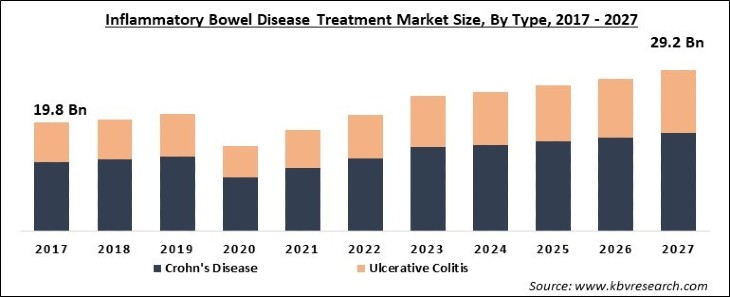 Source: KBV Research
Source: KBV Research
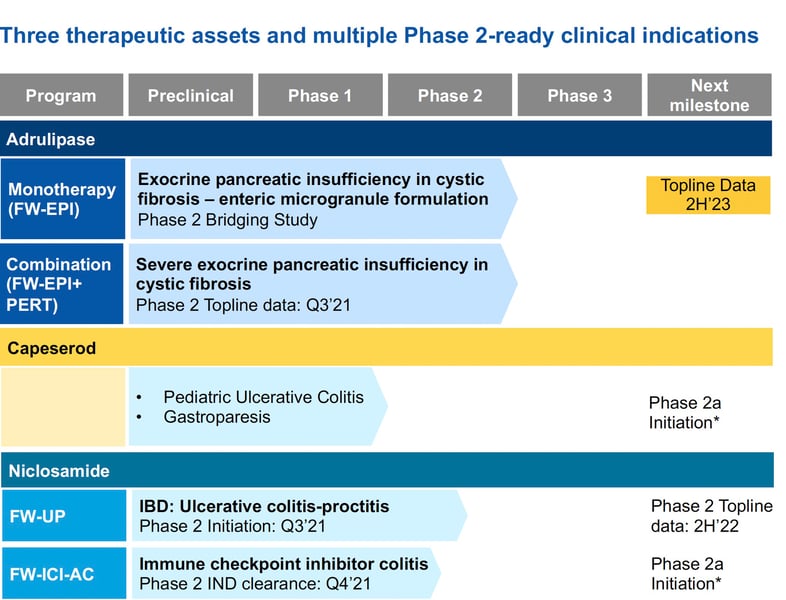
First Wave's lead product candidate is adrulipase, a recombinant enzyme being developed as an oral capsule for exocrine pancreatic insufficiency (EPI) in patients with cystic fibrosis and chronic pancreatitis. In January 2023, the company initiated a Phase 2b bridging study of an improved formulation of adrulipase. First Wave continues efforts to advance its pipeline of non-systemic GI therapies.
First Wave Pipeline

First Wave BioPharma is developing targeted, non-systemic therapies for gastrointestinal (GI) diseases. The company's pipeline focuses on advancing its lead product candidate, adrulipase.
Adrulipase is a recombinant lipase enzyme being developed as an oral capsule for treating exocrine pancreatic insufficiency (EPI) in patients with cystic fibrosis and chronic pancreatitis.
In January 2023, First Wave initiated a Phase 2b bridging study evaluating an improved enteric microgranule formulation of adrulipase in cystic fibrosis patients with EPI. While initial data from this study showed the new formulation was safe and improved over prior versions, efficacy results were inconclusive. Additional data is expected in Q3 2023.
Source: Company Reports
Adrulipase


Adrulipase is a recombinant lipase enzyme being developed by First Wave BioPharma as an oral capsule for treating exocrine pancreatic insufficiency (EPI) in patients with cystic fibrosis (CF) and chronic pancreatitis (CP).
EPI is a condition characterized by a deficiency of pancreatic digestive enzymes, resulting in a patient’s inability to digest food and absorb nutrients properly.
The current standard of care is porcine-derived pancreatic enzyme replacement therapy (PERT). However, PERTs have limitations, including a high daily pill burden and potential safety issues.
In developing adrulipase, First Wave aims to provide an effective, non-animal-derived therapy to control EPI while reducing the daily pill burden.
In January 2023, the company initiated a Phase 2b bridging study evaluating an improved enteric microgranule formulation of adrulipase in CF patients with EPI. Additional data is expected in Q3 2023 as First Wave continues working to optimize the adrulipase formulation.
Capeserod


Capeserod: Unique Mechanism of Action Applicable to New GI Indications
-
Capeserod, a selective 5-HT4 receptor partial agonist, was in-licensed from Sanofi in September 2023
-
In previous Sanofi Phase 1 and Phase 2 CNS trials involving over 600 patients, Capeserod appeared safe and well-tolerated
-
Research on Capeserod and subsequent artificial intelligence (AI)-empowered analyses suggest that the drug possesses a unique mechanism of action that is applicable to several GI indications underserved by currently available therapeutics in multi-billion dollar markets.
– Pediatric Ulcerative Colitis
– Gastroparesis
– Constipation
-
First Wave will repurpose Capeserod for gastrointestinal (GI) indications and plans to initiate a Phase 2 clinical development program
-
In September 2023, First Wave announced an agreement with Sanofi (NASDAQ: SNY) to license Capeserod.
Niclosamide


Niclosamide is an oral small molecule with anti-viral and anti-inflammatory properties that has been safely used on millions of patients worldwide. It is listed as an essential medicine by the World Health Organization (WHO) and was approved by the U.S. Food and Drug Administration (FDA) in 1982 for the treatment of intestinal tapeworm infections.
Recent discoveries in immune cell metabolism suggests that it is possible to selectively target disease-causing immune cells to treat inflammatory diseases without unwanted side effects such as broad immunosuppression. Research indicates that IBD, including ulcerative colitis, Crohn’s disease, and ulcerative proctitis/ proctosigmoiditis, is driven by pathogenic Th17 cells, which releases a cascade of local cytokines that in turn cause inflammation in bowel wall tissues. Th17 cells rely on a cellular process called oxidative phosphorylation to survive. Niclosamide is known to disrupt the oxidative phosphorylation in the mitochondria of pathogenic Th17 cells in a manner that selectively induces apoptosis of pathogenic Th17 cells, overcoming their inherent resistance to cell death. By killing Th17 cells, niclosamide reduces inflammation and calms the gut, selectively killing pathogenic, inflammatory cells while leaving healthy cells untouched.
First Wave’s suite of proprietary, gut-restricted niclosamide product candidates are designed to target the metabolism of disease-causing Th17 cells to potentially halt or delay the progression of disease, stop flare-ups, and address patient needs at all stages of IBD, from mild to severe, and for cancer patients with checkpoint-induced colitis.
Financials
SEC Filings
Management Overview

James Sapirstein
Chairman, President & CEO
Mr. Sapirstein has served for over thirty-nine years in the pharmaceutical industry. After spending seventeen years in large Pharma companies, Mr. Sapirstein started his career in smaller biotech companies. He later joined Gilead Sciences, Inc. to lead the Global Marketing team in launching Viread (tenofovir). In 2002, he accepted the position of Executive Vice President, Metabolic and Endocrinology for Serono Laboratories. Later, in 2006, he became the founding Chief Executive Officer of Tobira Therapeutics, then a private company. Tobira Therapeutics was acquired by Allergan in 2016. In 2012, Mr. Sapirstein became the Chief Executive Officer of Alliqua, Inc. Thereafter, he served as Chief Executive Officer of Contravir Pharmaceuticals from March 2014 until October 2018.
Mr. Sapirstein has raised over $400 Million in venture capital and public capital markets financing in his various engagements as Chief Executive Officer. He was named as a Finalist for the Ernst & Young Entrepreneur of the Year award in 2015 as well as in 2016. Mr. Sapirstein currently holds board positions on Enochian Biosciences, ZyVersa Therapeutics, and Blue Water Biotech. He was Chairman of the Board for BioNJ, an association of biopharma industries in New Jersey, from February 2017 to February 2019. In addition, he is a member of the Board of Directors for BIO (Biotechnology Innovation Organization), the leading biotechnology trade organization promoting public policy and networking in the healthcare space, where he sits on the Emerging Companies Section Governing Board and Health Section Board. Mr. Sapirstein received a BS (Pharmacy) from Rutgers University and an MBA from Fairleigh Dickinson University.
Sarah Romano
Chief Financial Officer
Sarah Romano, CPA, was appointed as Chief Financial Officer of First Wave BioPharma in March 2022. She previously served as Chief Financial Officer of Kiora Pharmaceuticals, Inc. (NASDAQ: KPRX) (formerly EyeGate Pharmaceuticals, Inc.), a clinical-stage specialty pharmaceutical company developing products for treating ophthalmic diseases, from February 2017 through February 2022, and as its Corporate Controller from August 2016 to January 2017. Prior to joining Kiora, Ms. Romano served as Assistant Controller at TechTarget from June 2015 through August 2016 and Corporate Controller at Bowdoin Group, a healthcare-focused executive recruiting firm, from September 2013 through May 2015. Previously, she held financial reporting positions of increasing responsibility at SoundBite Communications from 2008 until its acquisition by Genesys in 2013 and at Cognex Corporation from 2004 through 2008. Ms. Romano began her career as an Auditor in the Boston office of PricewaterhouseCoopers. A licensed CPA in Massachusetts, she holds a Bachelor of Arts in Accounting from the College of the Holy Cross and a Master of Accounting from Boston College.
Martin Krusin
Senior VP for Corporate Development
Mr. Krusin is an experienced executive with 20 years of business development, strategic marketing, financing, and operating experience in the healthcare and financial services sectors. Prior to joining First Wave BioPharma as VP for Business Development in 2014, Mr. Krusin was VP for BD at FluoroPharma Medical, Inc.; Director of Business Development at Clewed; an Experienced Commercial Leader at GE Capital in its Global Sponsor Finance, Healthcare Financial Services, and Capital Solutions units; Vice President of Marketing & Sales and Director of Business Development at Electro-Optical Sciences; and an analyst in the Emerging Markets Strategic Planning Group at Citigroup. Mr. Krusin received an MBA from Columbia Business School in finance and marketing, an MPhil. in political economy from Oxford University, and a BA in international relations from Swarthmore College.
Ted Stover
VP of Product Development
Mr. Stover joined First Wave BioPharma in 2020 as the Product Development Director to oversee CMC and Project Management. Prior to joining First Wave BioPharma, Mr. Stover spent 20 years focused on manufacturing operations and analytical method development to support all stages of pharmaceutical drug development. Most recently, Mr. Stover served as the Senior Director of Program Management for Biorasi after holding positions of Vice President of Manufacturing at SCI and Operations Manager for Prioria Robotics. Mr. Stover earned his MBA from the University of Florida.
Amy Chandler
VP of Regulatory Affairs, QA & Compliance
Ms. Chandler has over 25 years of experience in the medical device, pharmaceutical, and combination product industries, supporting CNS, cardiovascular, endovascular, consumer, and wound care product development and manufacturing. She began her career at Johnson & Johnson, where she held multiple positions of increasing levels of responsibilities within the Quality Assurance organization (QAU, QA, QC, QE, SQA). She has expertise in developing Quality Assurance and Regulatory Affairs organizations and systems throughout all stages of a product life cycle. In addition, she has created both US and international strategies and submissions to secure key regulatory approvals and ensure quality and compliance. Ms. Chandler holds a BS degree in Chemistry and an MS degree in Textile Technology and is RAC (Global) certified.
Risks & Disclosures

This communication is neither an offer to sell nor a solicitation of an offer to buy, nor a recommendation of any securities of the company mentioned herein.
First Wave BioPharma (the “Company”) and its counsel have reviewed the content of this page as well as the accompanying presentation (“Company Presentation”) displayed on this page. To the best of its knowledge, the Company does not believe this content to be misleading or inaccurate in any material respect, nor does it believe there are any material omissions with respect to such content. The Company does not believe the contents of the page or the Company Presentation to contain any non-public material information.
Information and opinions presented in the Company Presentation are provided by the Company, and b2i digital makes no representation as to their accuracy or completeness. The information contained on this page is not intended to constitute any form of advice, and the information provided is not intended to provide a sufficient basis on which to make an investment decision. It is not investment research, nor does it constitute a research recommendation, as it does not constitute substantive research or analysis. This information is not to be relied upon in substitution for the exercise of independent judgment.
Information, opinions and estimates contained on this page or in the Company Presentation reflect judgments by the Company as of the original date of publication by the Company and are subject to change without notice. Past performance should not be taken as an indication or guarantee of future performance, and no representation or warranty, express or implied is made regarding future performance.
A complete description of the risks and uncertainties relating to the Company and its securities can be found in the company's filings with the U.S. Securities and Exchange Commission available for free at www.sec.gov.
Information on this page may relate to penny stocks, which may also be referred to as low-priced stocks. Penny stocks are low-priced shares typically issued by small companies. Penny stocks involve greater than normal risk, they may be less liquid than other stocks (i.e., more difficult to sell), and there may be less reliable information available regarding such stocks. Investors in penny stocks should be prepared for the possibility that they may lose their entire investment.
B2I DIGITAL, Inc. is a marketing sponsor of the Roth 34th Annual Roth Conference. B2I DIGITAL, Inc. is not an affiliate of Roth Capital Partners, LLC (“Roth”) and is not authorized to represent or act on behalf of Roth ,in any capacity. Roth has not reviewed and approved the content contained on the b2idigital.com website.