Nanōmix Corp.
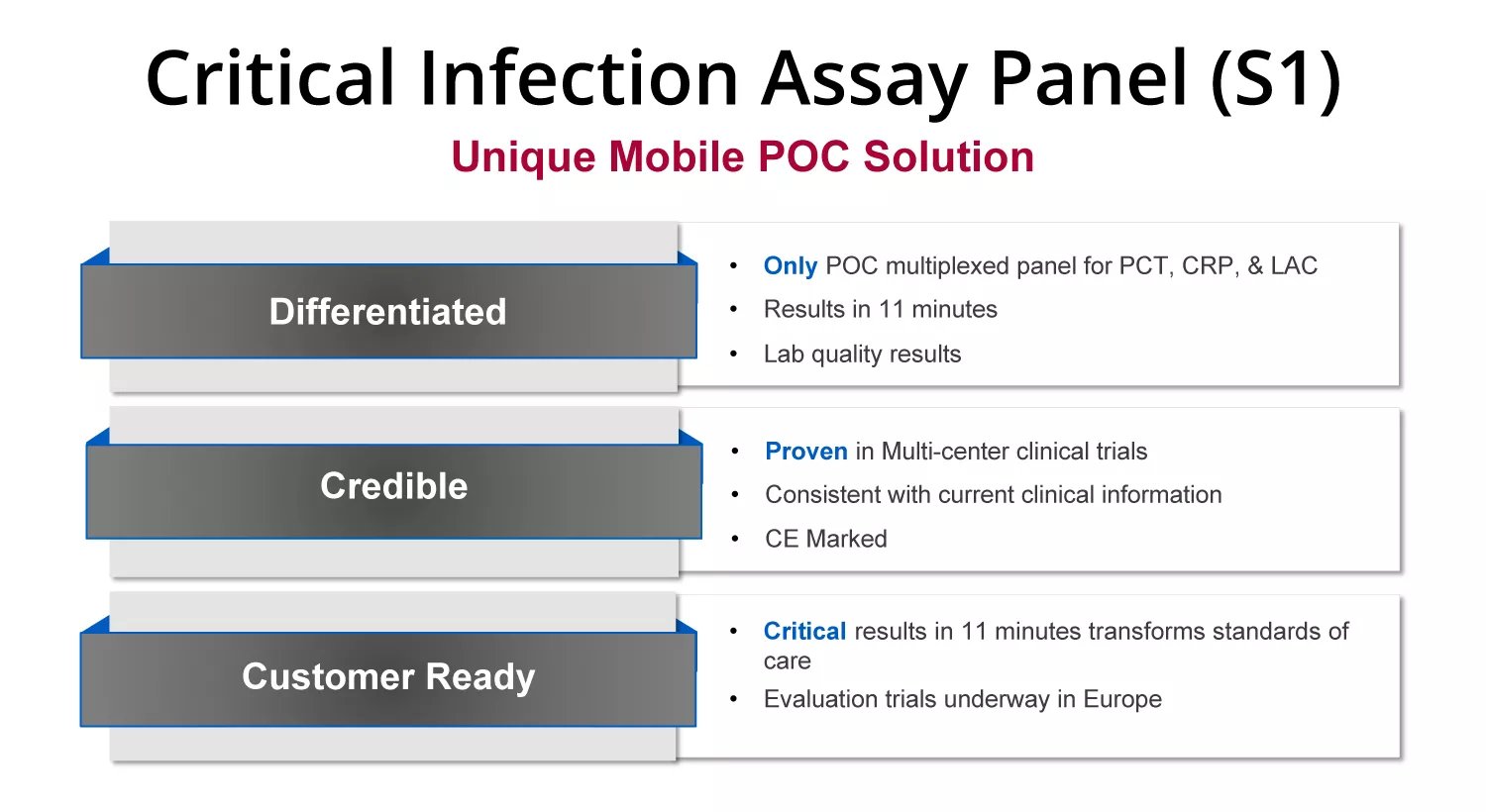
Nanōmix is a leader in decentralized testing and a participant in the large, fast-growing Point of Care (POC) diagnostics market. Their market-ready Critical Infection Panel is the only portable, rapid PCT assay and allows for obtaining critical information more quickly, leading to faster clinical decision-making.
OTCQB: NNMX
IR Website: https://ir.nanomixdx.com/
Headquarters: San Leandro, CA
Nanōmix has invested heavily into IP with all technology developed and wholly owned by the company, including 23 issued and pending patents. As a result they are moving towards a robust and connected future of one instrument with lab quality results for many different tests.
Last updated: 1/10/23
TALK TO MANAGEMENT
The Nanōmix management team is always available to talk to current and potential investors. They're happy to answer any questions you may have and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
Summary




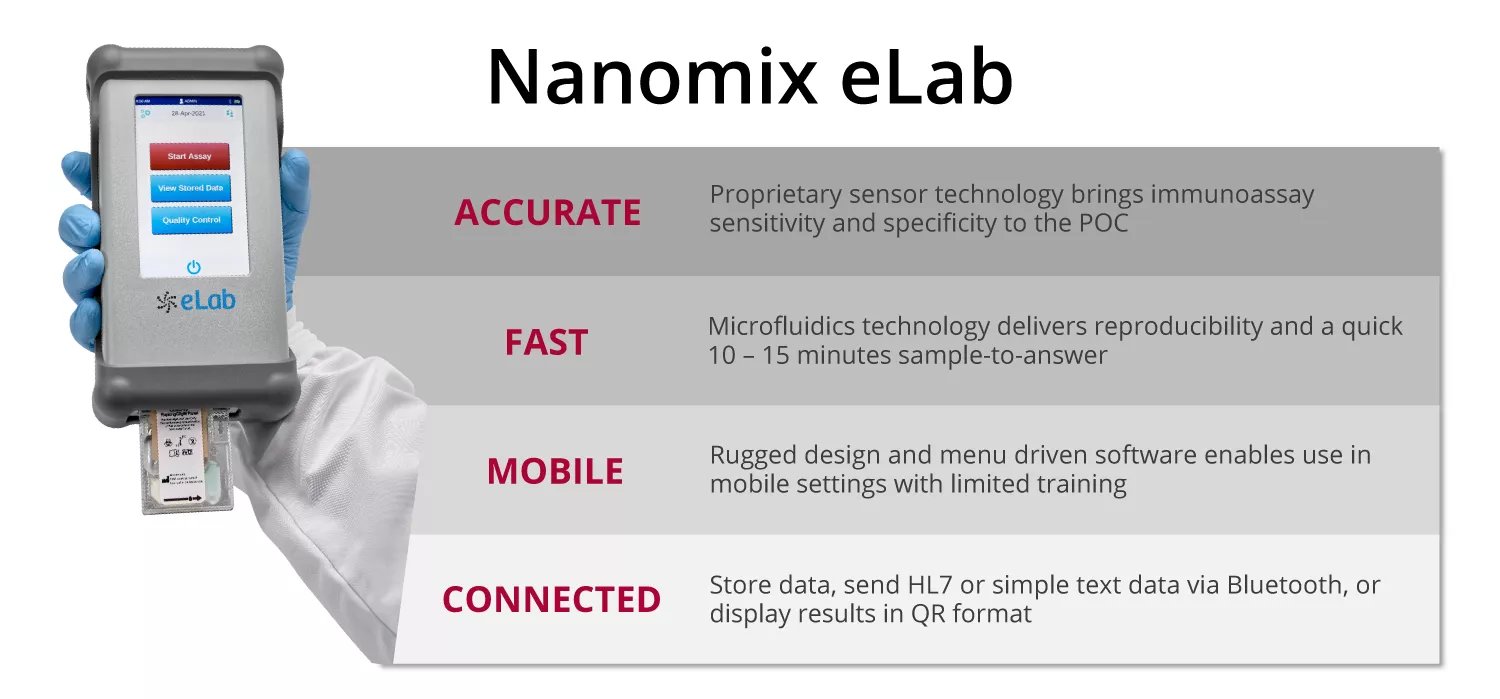
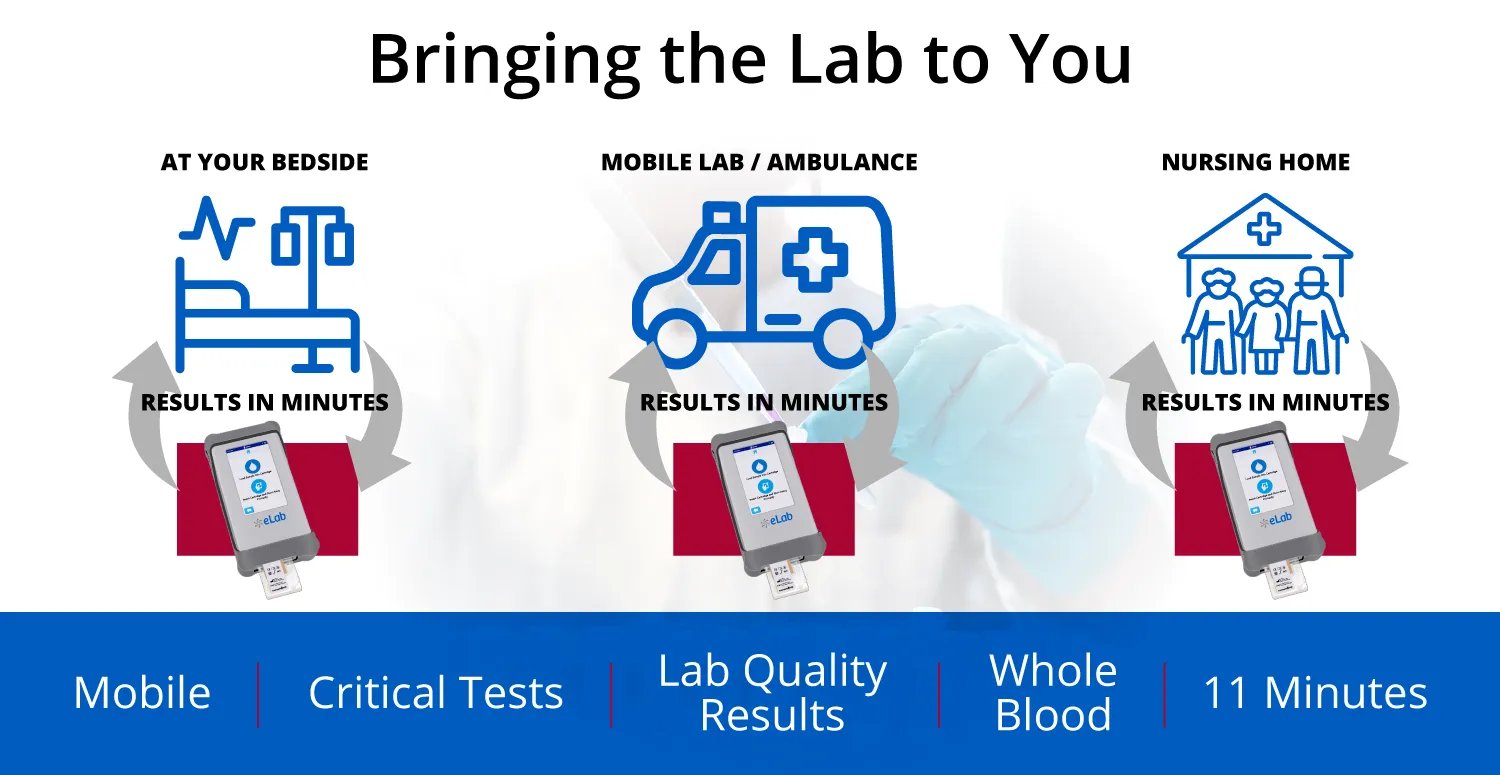
Nanōmix's eLab diagnostic system brings lab testing out of the hospital and to wherever the patient is, and aims to both disrupt a gigantic industry and save billions of dollars annually in the process.
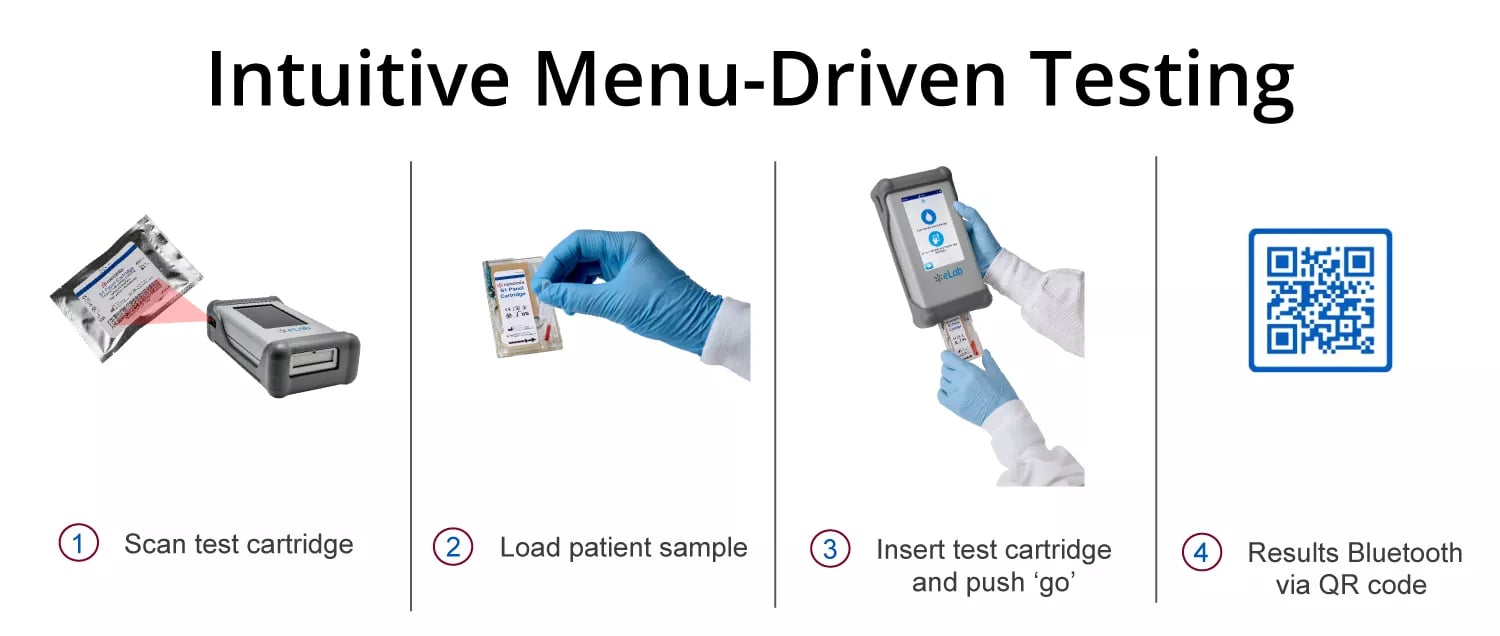
Nanōmix's Menu-Driven Testing capabilities already have the ability to aid clinicians in saving precious time in diagnosing deadly issues such as sepsis and show promise in their ability to diagnose myriad other health concerns in the future.
*The Nanōmix eLab® and S1 Panel Cartridge has not yet been FDA reviewed or cleared for sale in the U.S. It is not commercially available in the U.S. The Nanōmix eLab® and S1 Panel has received CE Mark and MHRA Registration. The Nanōmix eLab® COVID-19 Rapid Antigen Test is not commercially available in the U.S. The Nanōmix eLab® COVID-19 Rapid Antigen Test has received CE Mark. Other assays in development
Press Releases
Investor Presentation


To download the Nanomix investor presentation, please fill out the form below.
Stock Chart (Intraday)
Stock Chart (Historical)
Video: The Nanōmix Vision

Video: An Introduction to Nanōmix with Board Member and ex-CEO David Ludvigson

Video: Diving Deeper Into The Company Itself

Disrupting the Place and Time of Quality Diagnostics, Better Outcomes

Disrupting an Old and Inefficient Model
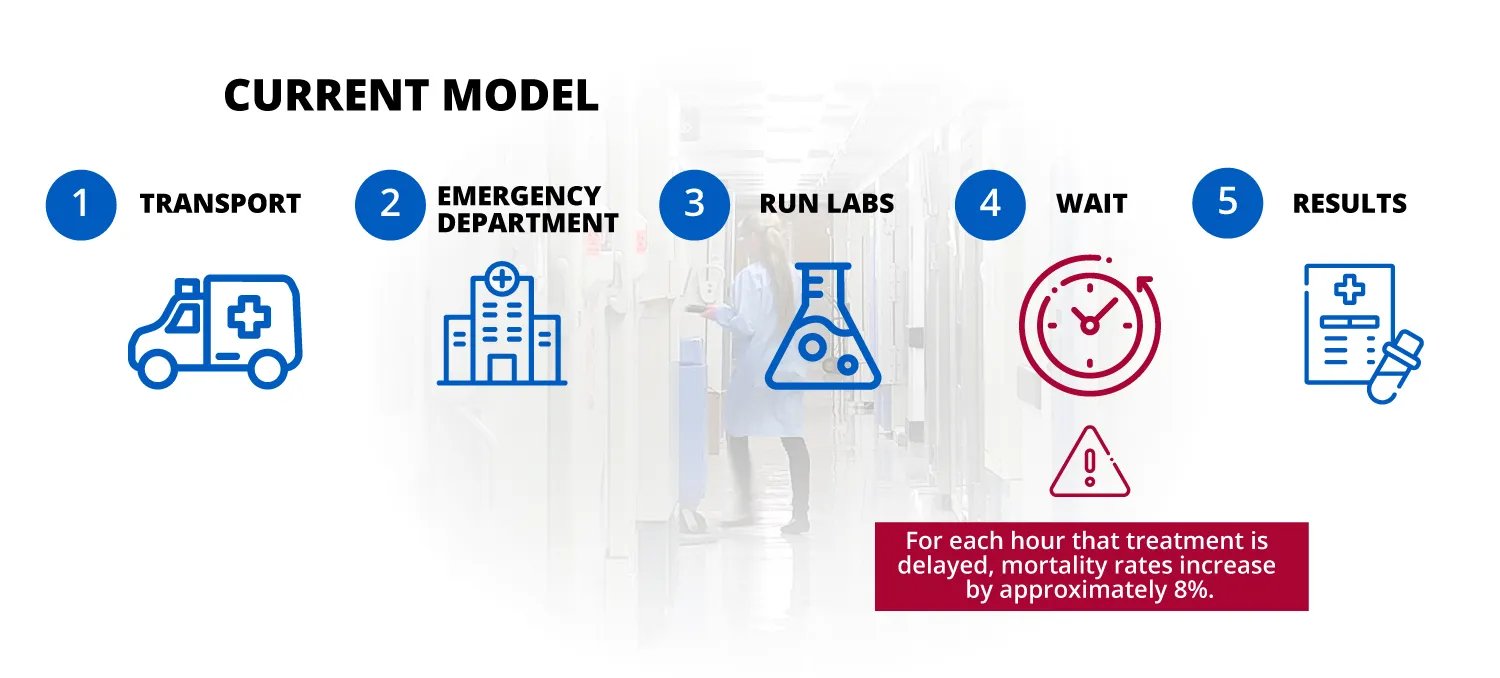
When you feel like something is wrong with your health, what are your options to figure out what is wrong? Typically go to the doctor's office or the hospital to get tests done. The result is not only inconvenient, but is also a major cost burden to the entire healthcare system and all stakeholders therein.
But what if the patient-clinician relationship, and with it the lab, could come to you? That is Point-of-Care Testing, and this is what Nanomix aims to bring to a larger market with its eLab analyzer and S1 panel.
Point-of-Care Testing with the Nanōmix eLab® System
Nanomix is the leader in the development of mobile point-of-care diagnostics, with a hand-held platform and assays that provide rapid, accurate, quantitative information for new use in settings where time is critical to clinical decision making and improved patient care. The company’s products are designed to broadly impact health care delivery by bringing diagnostics to the point of initial patient interaction, whether in the hospital or in pre-hospital, remote, or alternative settings, thus enabling faster clinical decision-making and potentially treatment-in-place.

Nanōmix Delivers On the Promise of Point of Care Testing | A $20B Market Ripe for Disruption
-
Leader in decentralized testing – large, fast-growing POC market
-
Need for decentralized testing is reinforced by COVID-19 experience
-
-
Market Ready Critical Infection Panel – faster critical information to speed clinical decision making
-
The ONLY portable, rapid system using a single, whole blood sample to detect multiple infection biomarkers PCT, CRP and LAC.
-
Drives unique rapid testing solutions for sepsis / pneumonia
-
-
Most Advanced Mobile Diagnostic System
-
One instrument for many different tests
-
Lab quality results in an easy-to-use system
-
Robust and connected
-
-
Deep Technical Expertise and IP
-
All technology developed and wholly owned by Nanōmix
-
23 issued and pending patents
-

Accessibility to the Quality of Our Technology
Platform Technology, Designed for Rapid Menu Expansion


-
Optimized for multi-modality tests (multiple types of test types within 1 cartridge)
-
Designed for multi-plex testing, allowing the right tests to be combined together in one cartridge
-
Standardized for future assay success: rapid optimization of proven chemistries while leaving core cartridge design the same
-
Development time is greatly reduced
-
Operational costs reduced while receiving the benefits of standardized scale
-
Allows development partners while protecting the IP of the cartridge and system
-
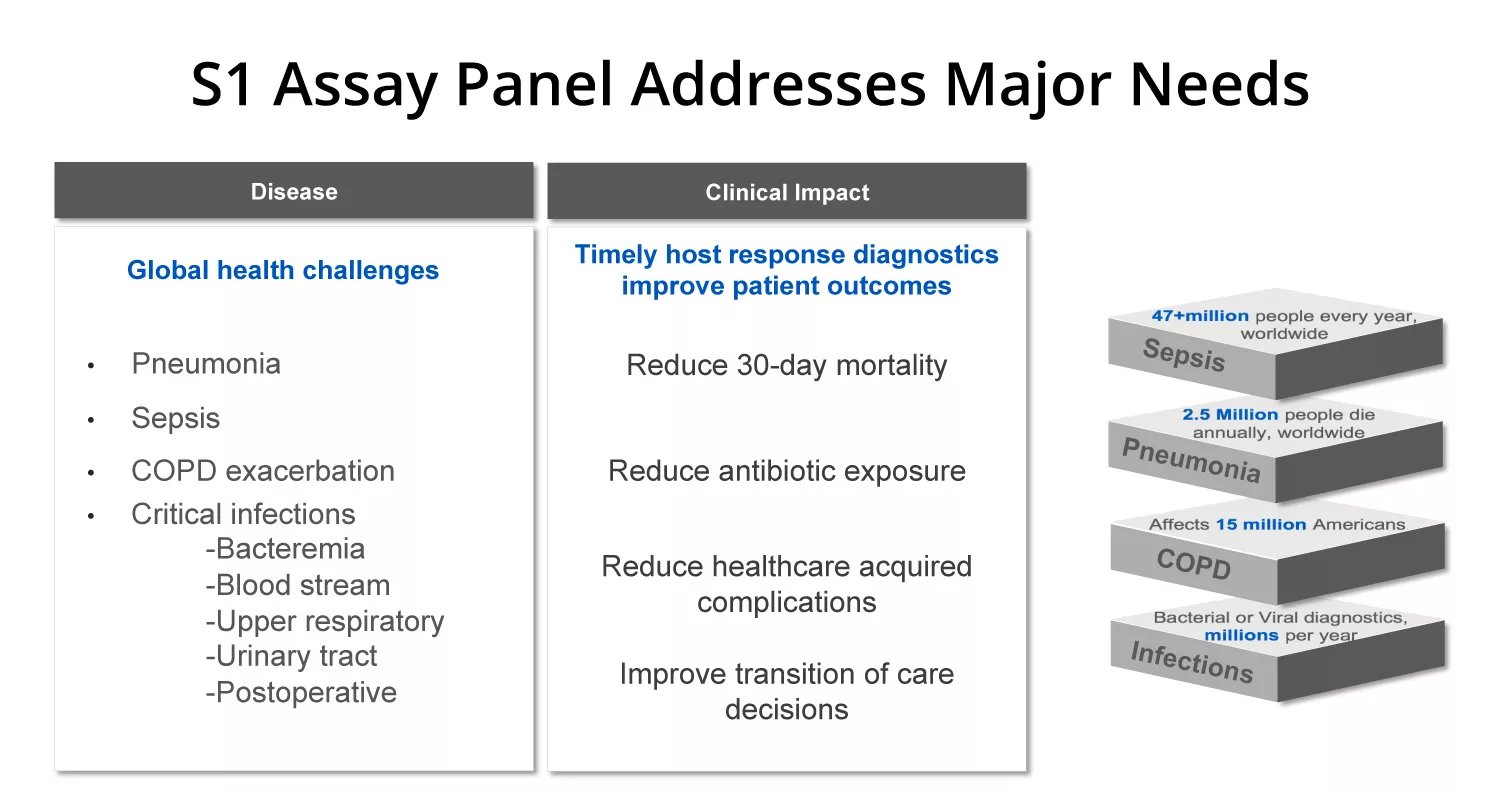
Addressing Major Market Needs

Informing critical diagnostics at the earliest point of patient interaction
Sepsis occurs when the immune system’s response to an infection spirals out of control. This can cause organ failure and other internal damage, and even death. There is an 7.6% increase in mortality for every hour of delayed treatment, so quick and accurate diagnosis is critical.
That’s why Nanōmix developed an 12-minute critical infection test to use with their handheld Nanōmix eLab analyzer. The test covers three key biomarkers — lactate, procalcitonin (PCT), and C-reactive protein (CRP) — to give healthcare professionals more comprehensive information to make a sepsis diagnosis. Those biomarkers were chosen as a result of wide global acceptance and thorough documentation in clinical studies and literature.
The Nanomix eLab® System aids clinicians in assessing
pneumonia, COPD exacerbation, and other critical infections. Nanomix is actively building out other diagnostic panels to aid in other critical indications.
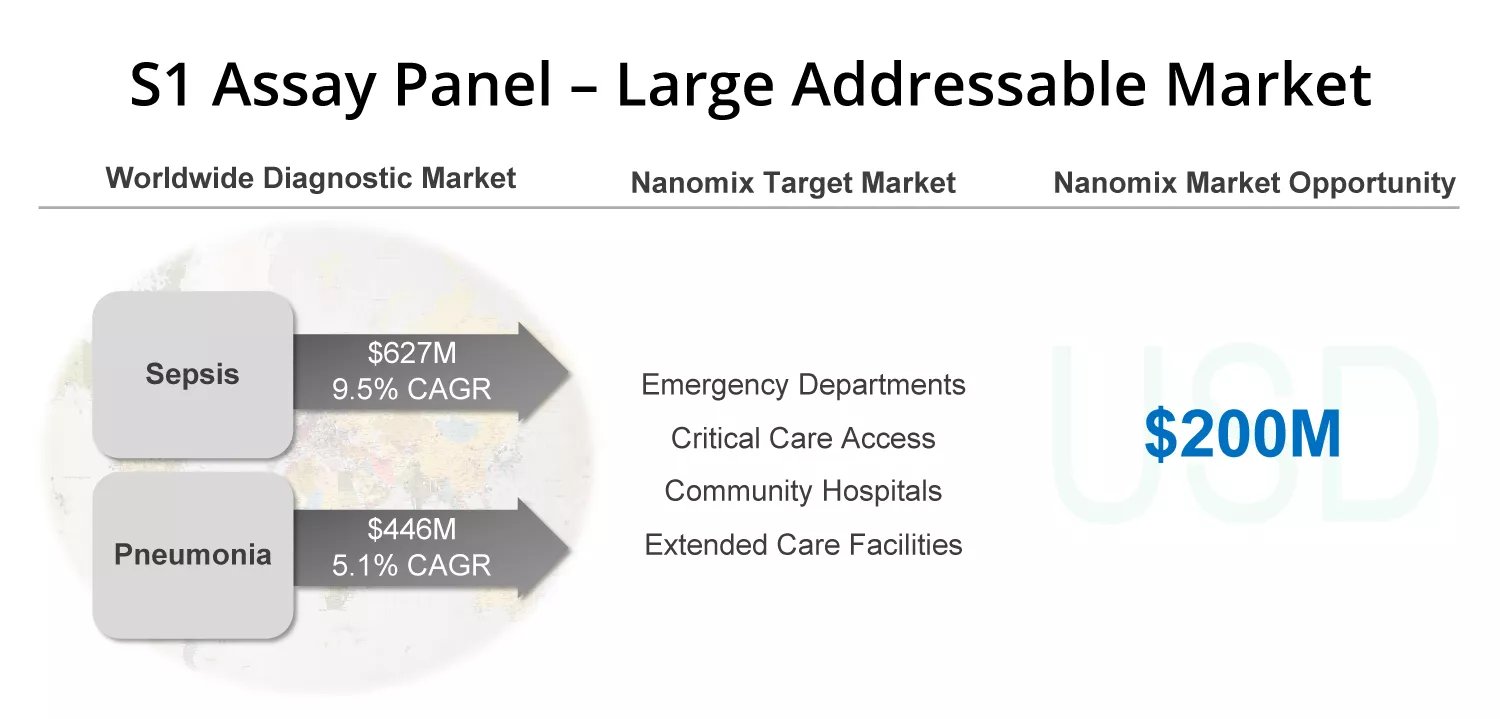
For sepsis and pneumonia alone, Nanōmix sees a $200 million market opportunity.
Expertise Matters

Nanōmix is Defined By its Leadership
The Chairman of the Board and largest shareholder of Nanōmix is Garrett Gruener. Gruener has been working for more than two decades in the fields of systems engineering and corporate development. In 1982, he founded Virtual Microsystems, a communications software company that he helped build into one of only two commercially available products that let VAX/VMS systems run standard off-the-shelf PC applications from terminals and VAXstations.

However, he is most well known as the founder of AskJeeves.com, now Ask.com, the search query website that served as the world's Google before Google became a verb. After that he co-founded Alta Partners, a venture capital firm in life sciences. He brings his expertise regarding building a successful tech company and scaling up effectively and efficiently.
While Garrett originally served as CEO, he understood that he needed a true diagnostics expert to overcome the scientific pitfalls that have overcome other similar companies, so he recruited and brought on David Ludvigson.

David is a financial and operating executive with over 35 years of international experience in life sciences and technology companies including IDEC Pharmaceuticals, Matrix Pharmaceutical, Nanogen, and MIPS Computer Systems. His experience over 15 years in the diagnostics arena has led numerous new product efforts from concept to market launch.
Lastly, David and the rest of the management team recognized the need to develop the next generation of management for the commercial growth phase of the company. With that in mind, David moved into an interim CFO role (while remaining on the Board of Directors) and passed the CEO baton to Dr. Thomas Schlumpberger.

Dr. Schlumpberger is a recognized expert in POC diagnostics and a seasoned senior executive with more than 20 years in the life sciences industry. He has successfully launched point-of-care products globally, as well as an ultra-high sensitivity assay in Europe. His broad base of experience is directly applicable to the next phase of growth at Nanōmix as the Company’s POC platform gains customer adoption.
Together they demonstrate both scientific leadership, as well as the ability to bring great products to market.
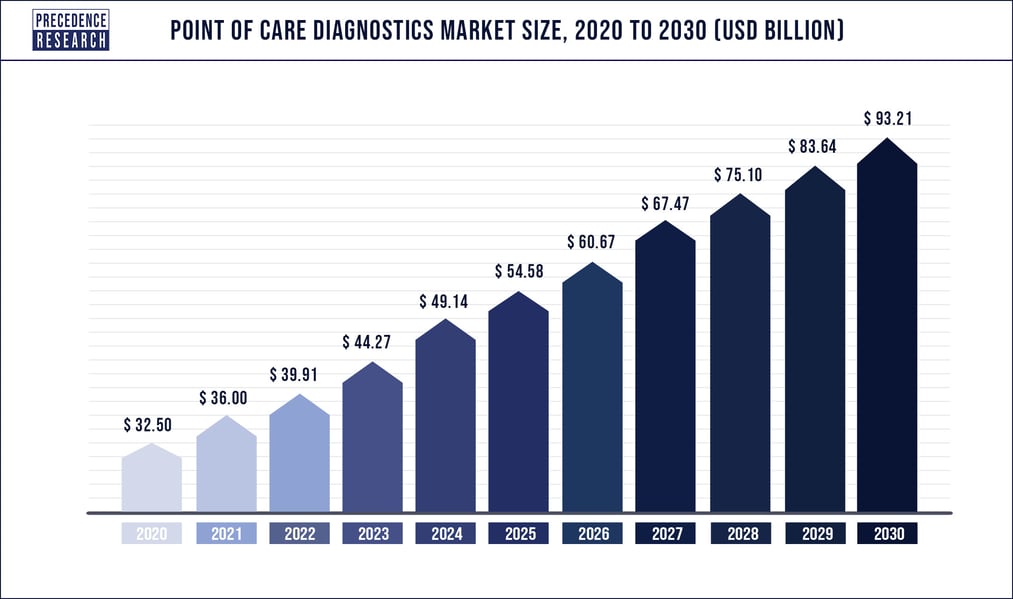
The Point-of-Care (PoC) Industry

According to Precedence Research, the global Point-of-Care diagnostics market was estimated to be $36 billion in 2021 and is predicted to increase to $93.2 billion in 2030, leading to a 11.1% Compound Annual Growth Rate over that time period.
The Institute of Medicine (IOM) estimates that $750 billion—30% of the U.S. annual health care budget—is wasted on unnecessary services, inefficient delivery, excessive administrative costs and prevention failures. Studies have reported that up to 34% of Medicare patients transported by EMS to an ED could have been safely treated in an alternative setting.
Nanōmix's Intellectual Property and Regulatory Clearance

Steeped in Expertise and Intellectual Property
In 2019 Nanōmix received CE Mark for their testing; a CE Mark signifies that it has undergone rigorous European Union compliance testing and is able to be sold with that designation. As of April 2022 it is currently undergoing FDA 510(k) review. It is currently the only test for the procalcitonin marker that can be performed outside of a laboratory.
They also have registration from the Medicines & Healthcare products Regulatory Agency (MHRA) of the United Kingdom for the Nanomix eLab system and the S1 Panel Cartridge.
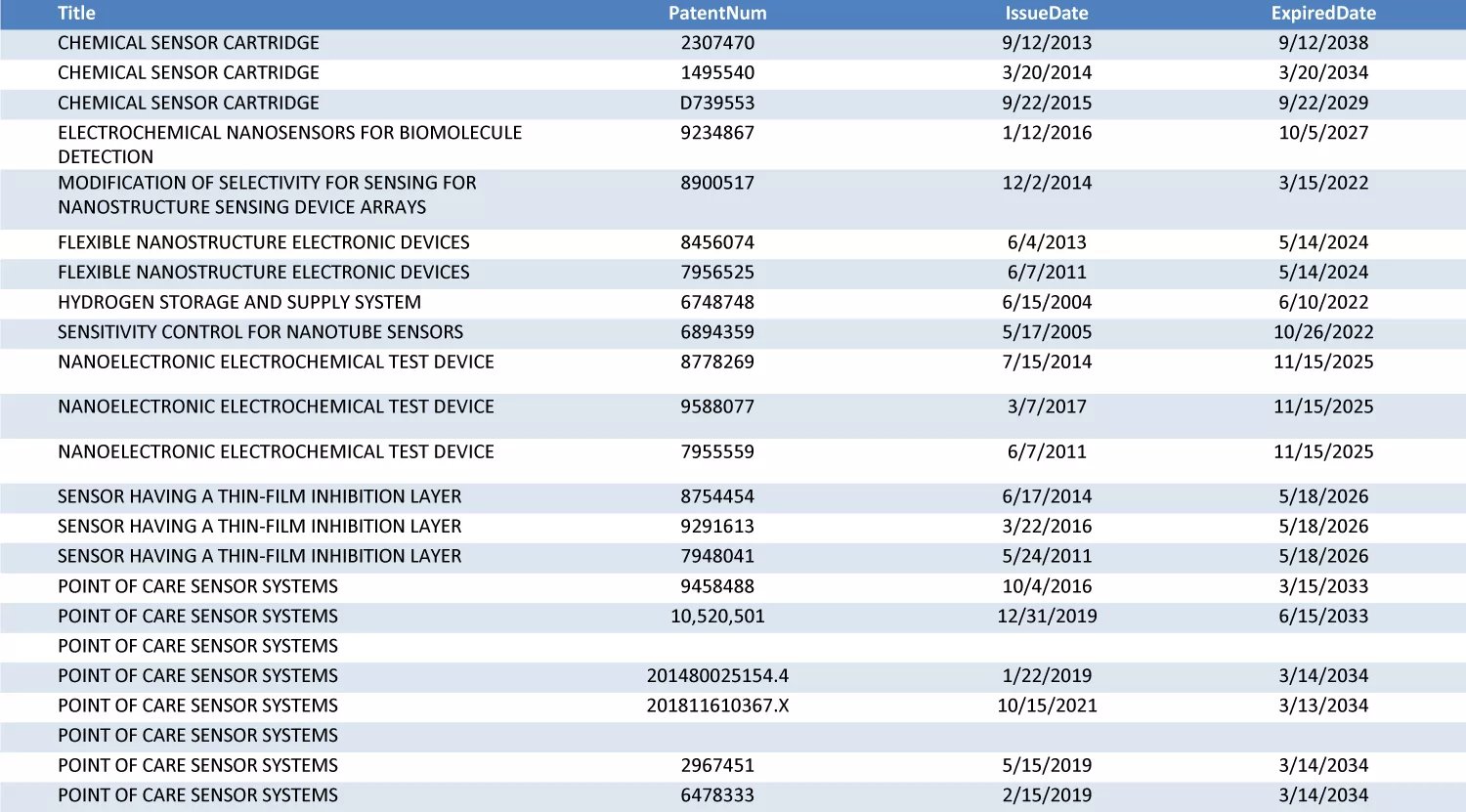
Nanōmix's patent portfolio can also be found below
Financials
SEC Filings
Management Overview


Thomas Schlumpberger
Chief Executive Officer
Dr. Schlumpberger is a recognized expert in POC diagnostics and a seasoned senior executive with more than 20 years in the life sciences industry. His broad base of experience is directly applicable to the next phase of growth at Nanōmix as the Company’s POC platform gains customer adoption. At Epocal, a POC company, he was responsible for international business and business development. He successfully launched a point-of-care product in India, Japan and Europe and, notably, he led the sale of Epocal to Alere. At Singulex, he concluded an investment from Grifols and successfully launched an ultra-high sensitivity assay in Europe. Most recently, as CEO of Pictor Limited, he successfully revamped the product portfolio and launched a COVID-19 antibody product successfully into several CLIA-certified laboratories. Dr. Schlumpberger has held senior executive roles with several other diagnostic companies, including Anixa, InVitae, Inivata, and Affymetrix. He received his Ph.D. in molecular and cell biology with distinction from the University of California, Berkeley.

Chris Hetterly
Chief Financial Officer
Mr. Hetterly is a financial executive and entrepreneur, with over 25 years as a CFO and technology banker with companies including Octagos Health, Madrona Ventures, Palo Alto Capital, GE Capital, Bank of America and Wells Fargo. He has raised capital from seed stage to IPO and beyond, and has experience in debt and equity financing, corporate development, and business development, with particular expertise in building finance teams and leading in high growth environments. Mr. Hetterly has raised over $10 billion in capital in his career for such well-known names as eBay, Netflix and Symantec amongst others, and his financing experience includes venture capital, corporate, mezzanine, lease, bank credit line, LBO, IPO and secondary public sources. He graduated from Brown University with a BA from the Honors Program.

John Hardesky
Chief Commercial Officer
John is an accomplished leader with over 20 years of commercialization experience in the clinical diagnostics market. John has built a strong track record of innovative concept to execution strategies that yield turnaround and growth results. While holding leadership positions in manufacturer and distribution, start-up through large companies, he launched over 20 different product platforms and value-based solutions to market. John is passionate about creating a culture of entrepreneurial spirited drive with execution confidence.

Vidur Sahney
Chief Operating Officer
Vidur brings more than 23 years of experience in operations, quality, validation, manufacturing, and research. His experience includes FDA Class III and CE product approvals and commercialization activities. He has set up and scaled production, technical operations, and supply chains in several companies, including ExThera Medical, JUUL Labs, Thoratec, and Loma Vista Medical. He is a graduate of San Francisco State University and was trained in Six Sigma Lean Manufacturing by Toyota at the NUUMI plant in Fremont, CA.

Sherrill Lavagnino
Vice President of Engineering
Sherrill has over 25 years of experience in developing software and integrated hardware systems. As Senior Director of Engineering at Cognex Corporation, Sherrill was responsible for the successful launch of highly integrated manufacturing and inspection systems. Prior to Cognex she held the position of Senior Software Engineer at Isys Controls. The author of numerous patents, Sherrill graduated from Smith College.

Bradley Johnson, PhD
Senior Director of Technology Development
Brad has more than 10 years of broad experience in biotechnology and biomedical devices. His primary focus is in vitro diagnostic methods, related instrumentation and technology including microfluidics and electro chemical biosensors. He has been instrumental in the development of technology underpinning the Nanomix platform. He received his Ph.D. in Bioengineering from University of California, Berkeley.
Risks & Disclosures

This communication is neither an offer to sell nor a solicitation of an offer to buy, nor a recommendation of any securities of the company mentioned herein.
Nanomix Corporation (the “Company”) and its counsel have reviewed the content of this page as well as the accompanying presentation (“Company Presentation”) displayed on this page. To the best of its knowledge, the Company does not believe this content to be misleading or inaccurate in any material respect, nor does it believe there are any material omissions with respect to such content. The Company does not believe the contents of the page or the Company Presentation to contain any non-public material information.
Information and opinions presented in the Company Presentation are provided by the Company, and makes no representation as to their accuracy or completeness. The information contained on this page is not intended to constitute any form of advice, and the information provided is not intended to provide a sufficient basis on which to make an investment decision. It is not investment research, nor does it constitute a research recommendation, as it does not constitute substantive research or analysis. This information is not to be relied upon in substitution for the exercise of independent judgment.
Information, opinions and estimates contained on this page or in the Company Presentation reflect judgments by the Company as of the original date of publication by the Company and are subject to change without notice. Past performance should not be taken as an indication or guarantee of future performance, and no representation or warranty, express or implied is made regarding future performance.
A complete description of the risks and uncertainties relating to the Company and its securities can be found in the company's filings with the U.S. Securities and Exchange Commission available for free at www.sec.gov.
Information on this page may relate to penny stocks, which may also be referred to as low-priced stocks. Penny stocks are low-priced shares typically issued by small companies. Penny stocks involve greater than normal risk, they may be less liquid than other stocks (i.e., more difficult to sell), and there may be less reliable information available regarding such stocks. Investors in penny stocks should be prepared for the possibility that they may lose their entire investment.
b2i digital or its related entities may own securities of the Company.
The Company is a client of b2i Digital. The Company agreed to pay b2i Digital no greater than $100,000 in cash for 12 months of digital marketing consulting and investor awareness services.
*The Nanōmix eLab® and S1 Panel Cartridge has not yet been FDA reviewed or cleared for sale in the U.S. It is not commercially available in the U.S. The Nanōmix eLab® and S1 Panel has received CE Mark and MHRA Registration. The Nanōmix eLab® COVID-19 Rapid Antigen Test is not commercially available in the U.S. The Nanōmix eLab® COVID-19 Rapid Antigen Test has received CE Mark. Other assays in development
The Nanomix management and investor relations team is available to talk to current and potential investors. They're happy to answer your questions and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
• Directly hear the Nanomix story
• Ask your questions
• Submit the form below and someone will get in touch with you as soon as possible
Note: Company management or its representative can only discuss and disclose information that is already available in the public domain. They will do their best to clarify such information to the extent permitted by securities law and industry regulations.