
BioRestorative Therapies, Inc.
BioRestorative Therapies is an emerging growth biopharma company focused on the research, development and commercialization of cellular therapies.
Nasdaq: BRTX
IR Website: https://www.biorestorative.com/ir/
Headquarters: Melville, NY
The company's first platform technology is focused on musculoskeletal health; it is developing an indication using an autologous cell based therapeutic product to address chronic degenerative disc disease and has received approval from the FDA to initiate a Phase 2 clinical trial. Its second platform technology addresses metabolic disease through an allogeneic cell based therapeutic product derived from Brown Fat Tissue and has indications in obesity, Type 2 Diabetes, and PCOS amongst other metabolic diseases. The Company has a robust pipeline of indications that will be leveraged across its two primary platform technologies.
TALK TO MANAGEMENT
The BioRestorative, Inc. management team is always available to talk to current and potential investors. They're happy to answer any questions you may have and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
BioRestorative Therapies At-A-Glance

BRTX-100 and Chronic Lower Back Pain
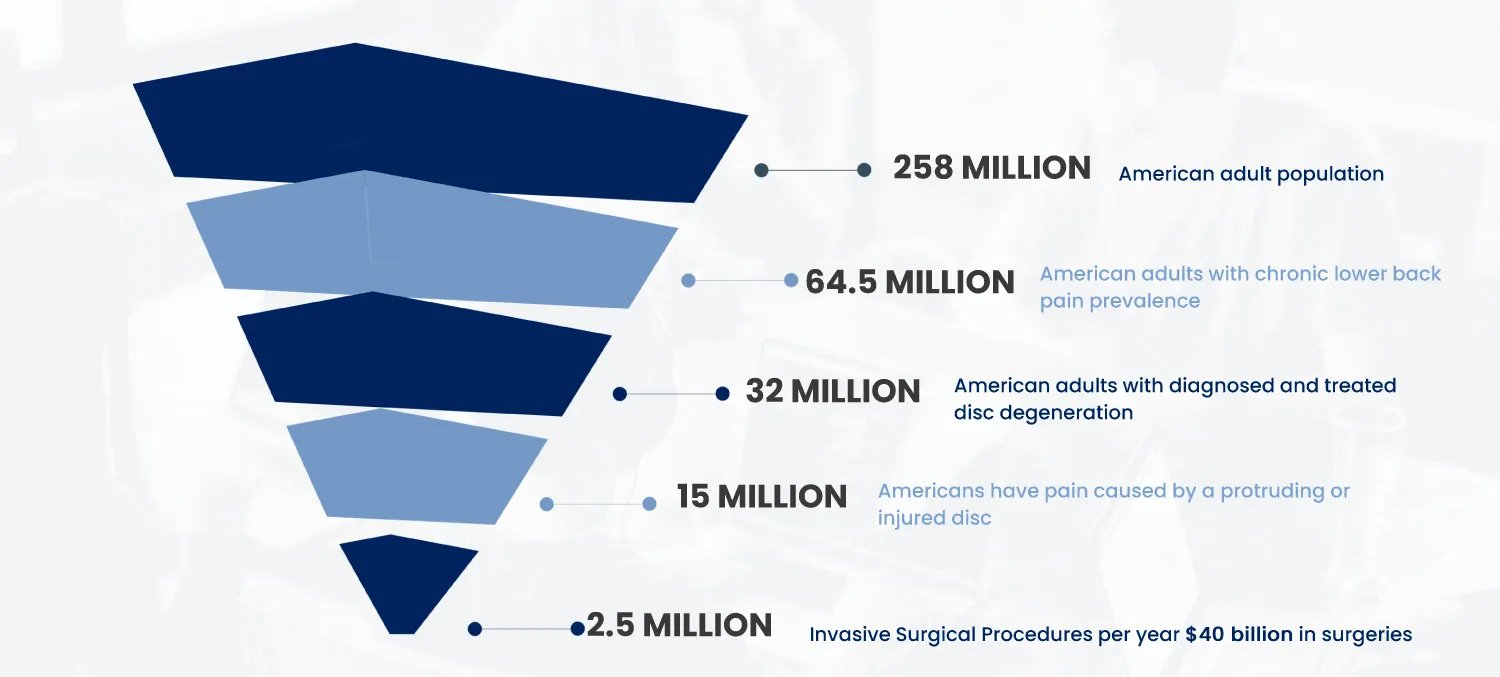
In the United States, 25 million people suffer from chronic lower back pain each year, half of whom have or will be diagnosed with disc degeneration. Current treatments–including opioid pain relievers, steroid injections, and physical therapy–simply manage pain, ignoring the root causes of the problem.
Metabolism and Fighting Fat
Obesity, diabetes, hypertension, hyperlipidemia and certain heart related disorders, have long been seen as contributing to a pandemic in the US—40% of Americans are obese; 30% have type 2 diabetes or pre-diabetes, and half of Americans have at least one major risk factor for heart disease.
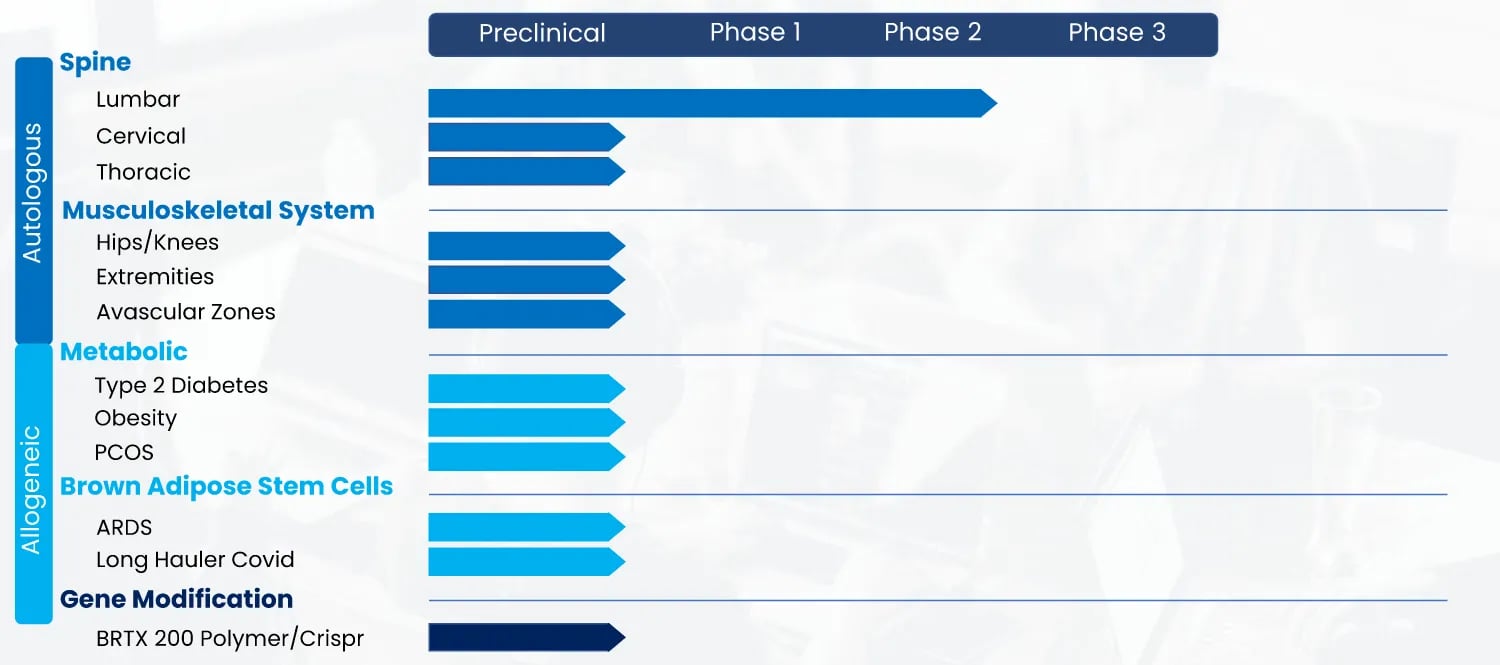
A Robust Pipeline for Myriad of Indications

BioRestorative Therapies was founded by scientists and clinicians committed to developing stem cell therapies to address unmet needs in patients with highly prevalent conditions.
Our advances in stem cell biology and delivery protocols harbor great promise in conditioning our bodies’ own regenerative potential to treat major diseases more effectively than current interventions.
Today, BioRestorative is actively developing programs that aim to dramatically increase quality of care for both (i) chronic back pain caused by disc degeneration, as well as (ii) metabolic disorders including obesity and diabetes.
BioRestorative Therapies believes that its lead drug, BRTX-100 will have a transformative impact on treating lower back pain. It is currently in Phase 2 trials for lumbar disc disease and is in preclinical trials for other back-related indications.

Additionally, its Thermostem program is designed to help fight obesity. Type 2 diabetes and other metabolic disorders.
Video: Interview with CEO Lance Alstodt

Video: BioRestorative Therapies underway with Phase 2 clinical trial with stem cell-based BRTX-100

Investor Presentation


To download the BioRestorative Therapies investor presentation, please fill out the form below.
Press Releases
Stock Chart (Intraday)
Stock Chart (Historical)
SEC Filings
Financials
BRTX-100 and Chronic Lower Back Pain

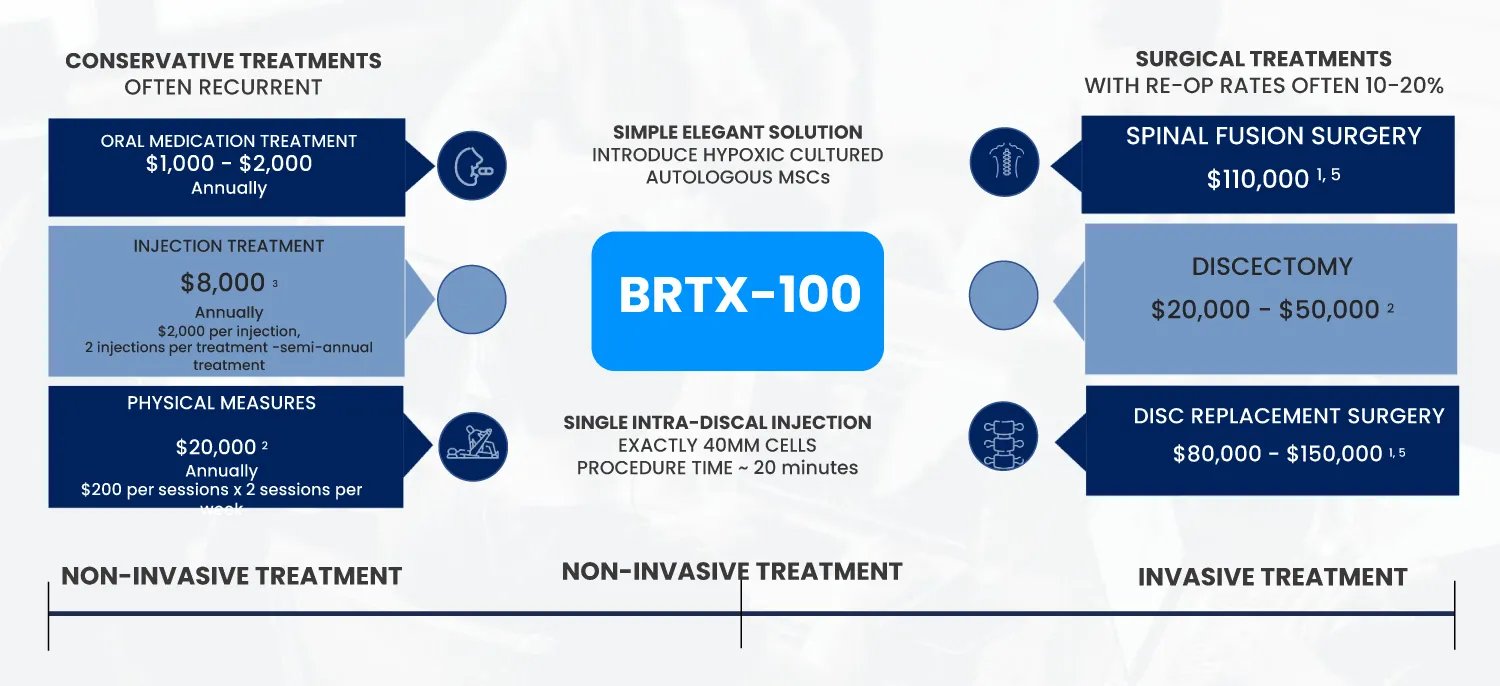
In the United States, 25 million people suffer from chronic lower back pain each year, half of whom have or will be diagnosed with disc degeneration. Current treatments–including opioid pain relievers, steroid injections, and physical therapy–simply manage pain, ignoring the root causes of the problem. Many patients reluctantly progress to surgical interventions, which are highly invasive and clinical outcomes have demonstrated limited, if any, therapeutic benefits.
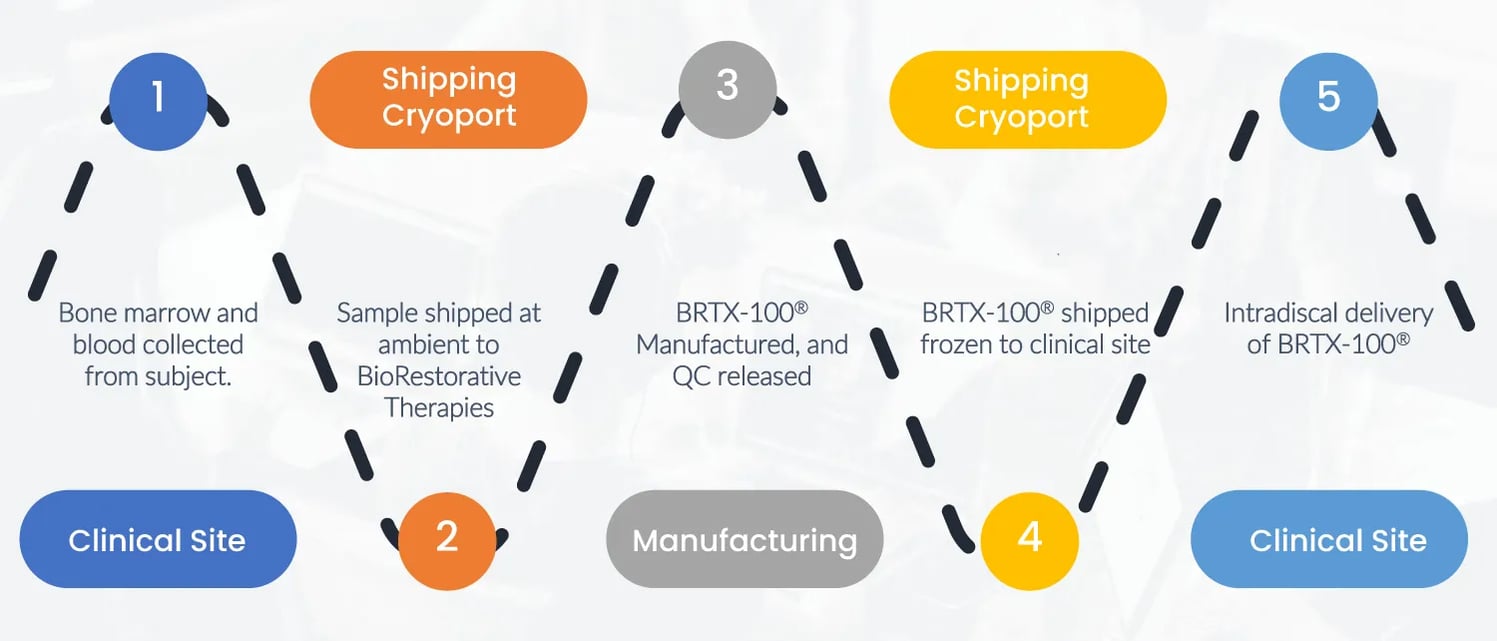
BRTX-100 is a novel product to treat damaged, degenerating discs, and is anticipated to be safer, cheaper, and more effective upon a single treatment. Specifically, BRTX-100 is an autologous stem cell product that uses your own stem cells that are harvested, cultured, and then injected directly into the affected disc to start the repair process.
These cells are created in a low-oxygen environment which transforms the cell into one with enhanced inflammatory markers, enhanced circulation, and enhanced regenerative and remodeling capability of the extracellular matrix.
BioRestorative Therapies has received authorization from the Food and Drug Administration to commence a Phase 2 clinical trial using BRTX-100 to treat persistent lower back pain due to painful degenerative discs.

Metabolism and Fighting Fat

Obesity, diabetes, hypertension, hyperlipidemia and certain heart related disorders, have long been seen as contributing to a pandemic in the US—40% of Americans are obese; 30% have type 2 diabetes or pre-diabetes, and half of Americans have at least one major risk factor for heart disease.
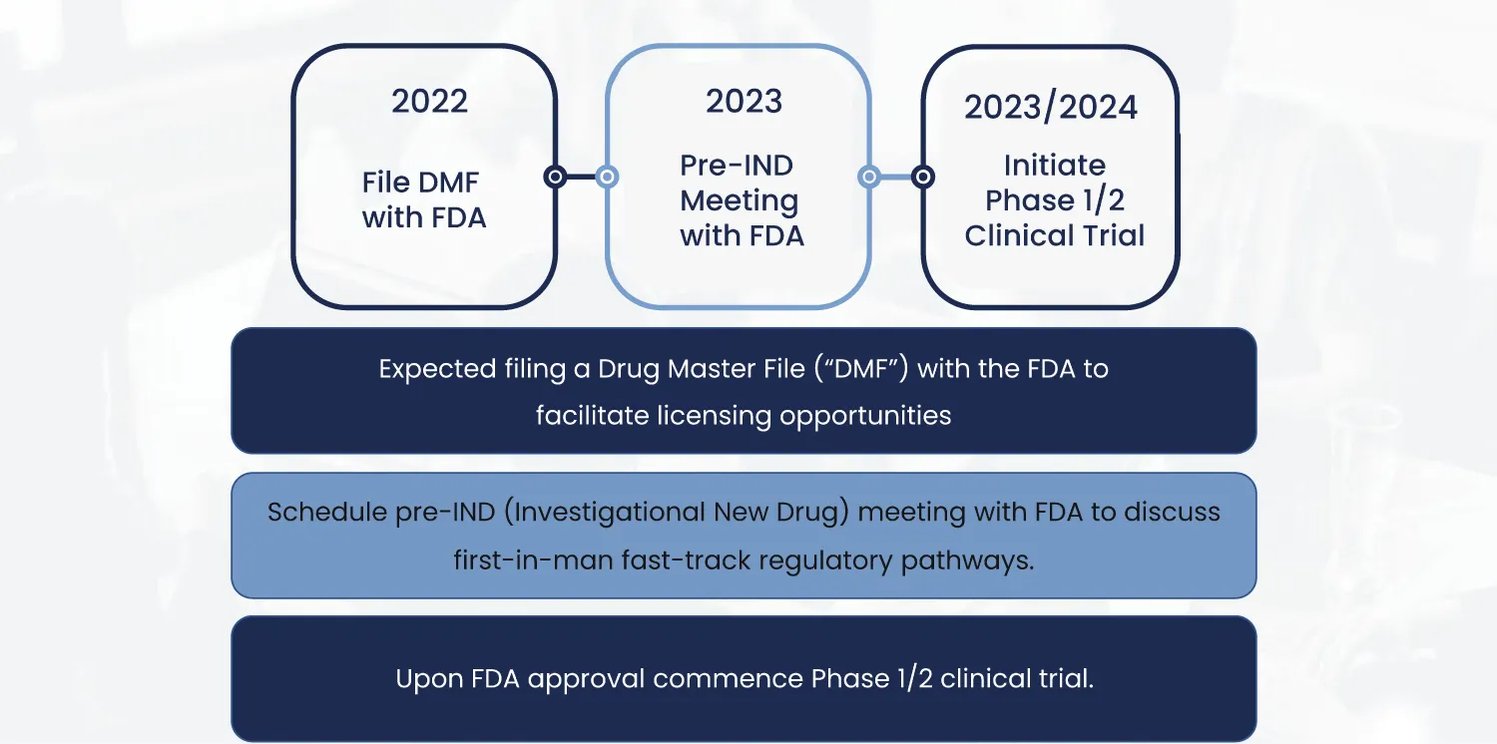
To target obesity and metabolic disorders, BioRestorative Therapies developed their ThermoStem® program that uses brown adipose-derived (“brown fat”) stem cells to generate new brown fat tissue. It was one of the first companies to identify and publish data about this novel stem cell population. This population of fat is known to burn, rather than store, energy. Elevated levels of brown fat have been demonstrated to increase metabolism and facilitate weight loss.
Initial preclinical research indicates that increased amounts of activated brown fat in the body may be responsible for additional caloric burning, as well as reduced glucose and lipid levels. Further, researchers have found that people with higher levels of brown fat may have a reduced risk for obesity and diabetes.
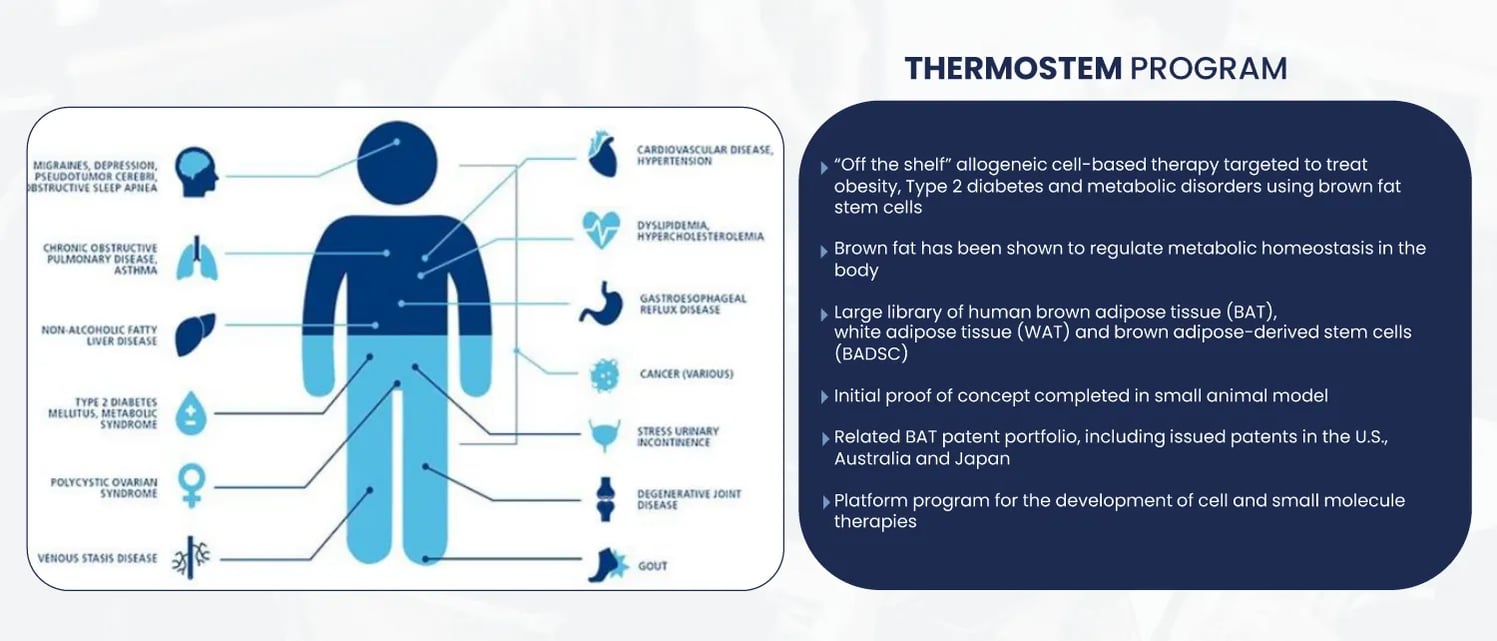
While this program is in relatively early stages, with a Pre-IND meeting with the FDA scheduled for 2023, it has already shown incredible promise.
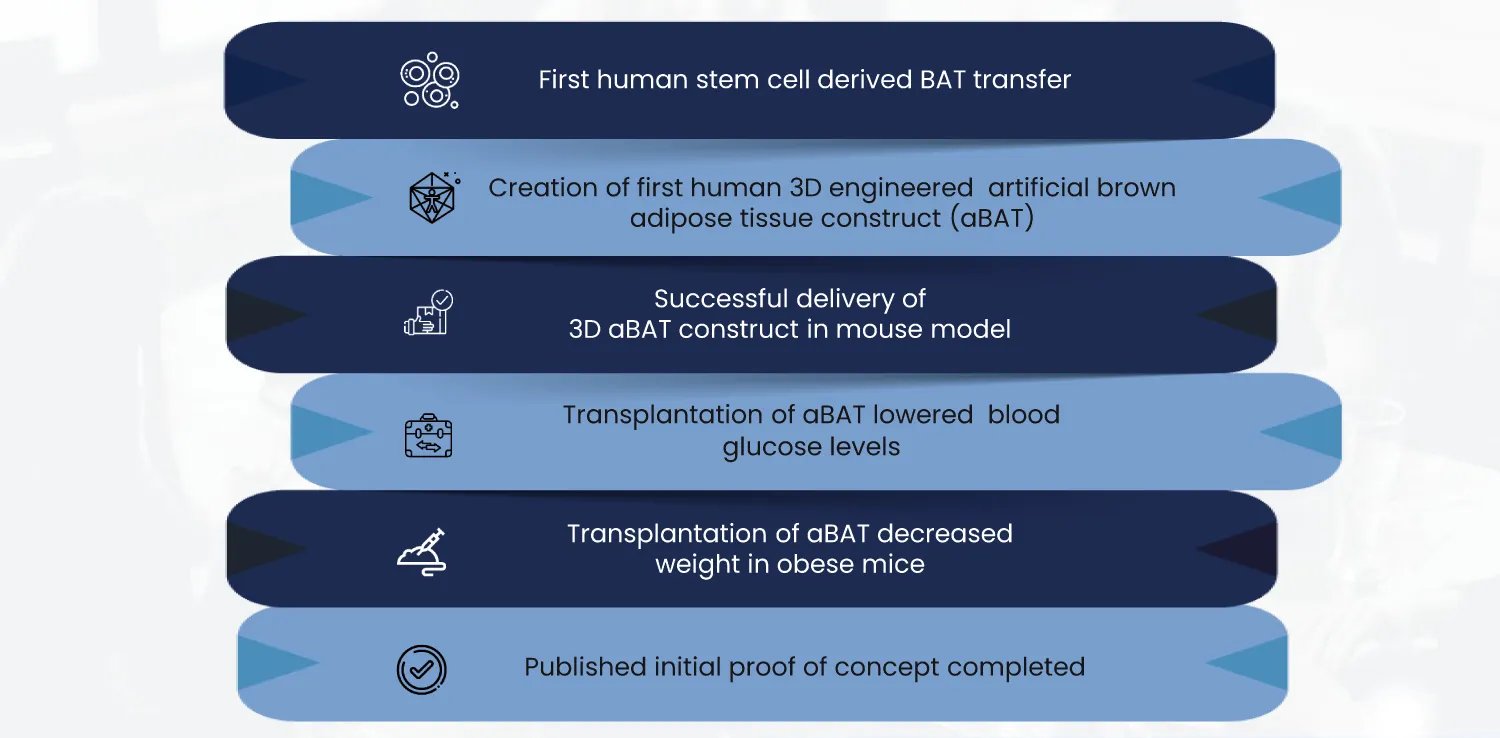
Economic Value Add

BioRestorative Therapies is creating solutions for two very significant global problems, both with strong potential for creating economic and shareholder value.
The disc degeneration opportunity alone is sizable and Biorestorative is sitting at the intersection between the $40 billion and 2.5 million patient invasive surgery market and the $32 billion conventional pain management non invasive market (32 million Americans have been diagnosed with and treated for disc degeneration, averaging around $1,000 per year for oral pain management).
Additionally, we all know that obesity is a pressing issue in many countries around the world. How much of an issue is it? According to Reports and Data, The global obesity treatment market was valued at $8.4 billion USD in 2020 and is expected to reach $27.08 billion USD by year 2028, leading to a CAGR of 15.7%.
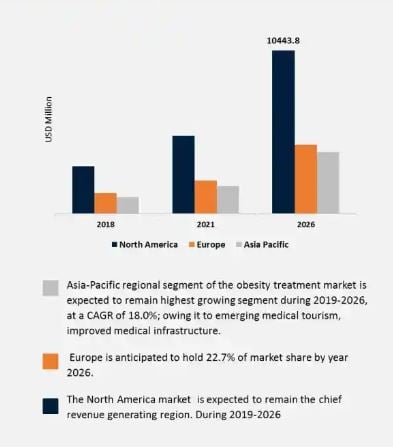
BioRestorative Therapies is positioning themselves well to create significant economic value in the treatment of these two pressing problems.
Management Overview - A Proven Management Team

Experience Matters
When it comes to drug commercialization, a wide variety of different skill sets and knowledge bases are needed. While the science and the location of an unmet need are obviously critical, much more is needed. The ability to effectively raise capital to create a long enough runway is critical, as is the ability to create and execute on a go-to-market strategy and strong knowledge of the various regulatory frameworks. BioRestorative Therapies' management team is incredibly well versed on all of these issues, well positioning them to succeed.

Lance Alstodt
President, CEO & Chairman of the Board
Lance Alstodt has been appointed the Company’s President, Chief Executive Officer and Chairman of the Board as of Nov 16, 2020. Mr. Alstodt brings over 25 years of experience in leading medical technology and lifesciences companies in operations, capital raising activities, strategy, and mergers and acquisitions.
Most recently, Mr. Alstodt was the Founder and CEO of MedVest Consulting Corporation (“MedVest”), an advisory and capital firm focused exclusively within the healthcare sector, focusing on growth and channel strategy, strategic planning, merger and acquisition support and investor activities.
Prior to MedVest, Mr. Alstodt was a career investment banker with over 25 years of experience in healthcare investment banking, including mergers and acquisitions. In 2011, Mr. Alstodt joined Leerink Partners as Managing Director to help lead its medical technology sector. Mr. Alstodt brings significant domain experience within the orthopedic and spine specific sectors. From 2008-2011, Mr. Alstodt was a Managing Director and Head of Medical Technology at Oppenheimer & Co. From 2000-2008, he was a Managing Director in the Healthcare Group and Global M&A Group at Bank of America Merrill Lynch (“BAML”). Prior to BAML, Mr. Alstodt spent seven years in the Global M&A Group at J.P. Morgan Chase, where he worked extensively on acquisitions, leveraged buyouts, private and public financings, exclusive sales and general advisory assignments.
Mr. Alstodt received a B.A. in Economics from the State University of New York at Albany, with a secondary concentration in Finance and Marketing.

Robert Kristal
Chief Financial Officer
Robert Kristal has been appointed Chief Financial Officer as of November 2021. Mr. Kristal brings an extensive array of strategic and financial markets experience to the Company, including a background in advising global public life sciences companies in corporate finance, operations management systems, and strategic collaborations.
Mr. Kristal is an experienced and versatile Wall Street and Bay St. professional of over 25 years. He has built teams in both institutional sales and equity research at firms which have developed a notable presence in healthcare research and capital market activities. Most recently he served as the Head of Research for H.C. Wainwright, growing their research product and presence in the biotech/biopharma space.
Mr. Kristal has been involved in numerous transactions in investment and merchant banking and has extensive experience in providing strategic advice and dealing with investors and corporate management

Francisco Silva
Chief Scientist & VP, R&D
Francisco Silva joined BRT in April 2011 and is Vice President of Research and Development. Mr. Silva is responsible for all laboratory operations and is involved in the development and growth of our stem cell programs.
Mr. Silva previously served as Chief Executive Officer of two companies engaged in the commercialization of human-based biologics for both research and therapeutic applications.
From 2003 to 2007, Mr. Silva was Vice President of Research and Development for PrimeGen Biotech LLC, a company engaged in the development of cell-based platforms. He was responsible for the development of experimental designs that focused on germ line reprogramming stem cell platforms.
Mr. Silva has taught courses in biology, anatomy and advanced tissue culture at California State Polytechnic University. He has obtained a number of patents relating to stem cells and has had numerous articles published with regard to stem cell research.
Mr. Silva graduated from California State Polytechnic University with a degree in Biology. He also obtained a Graduate Presidential Fellowship and MBRS Fellowship from California State Polytechnic University.
Risks & Disclosures

This communication is neither an offer to sell nor a solicitation of an offer to buy, nor a recommendation of any securities of the company mentioned herein.
BioRestorative Therapies Inc (the “Company”) and its counsel have reviewed the content of this page as well as the accompanying presentation (“Company Presentation”) displayed on this page. To the best of its knowledge, the Company does not believe this content to be misleading or inaccurate in any material respect, nor does it believe there are any material omissions with respect to such content. The Company does not believe the contents of the page or the Company Presentation to contain any non-public material information.
Information and opinions presented in the Company Presentation are provided by the Company, and b2i Digital makes no representation as to their accuracy or completeness. The information contained on this page is not intended to constitute any form of advice, and the information provided is not intended to provide a sufficient basis on which to make an investment decision. It is not investment research, nor does it constitute a research recommendation, as it does not constitute substantive research or analysis. This information is not to be relied upon in substitution for the exercise of independent judgment.
Information, opinions and estimates contained on this page or in the Company Presentation reflect judgments by the Company as of the original date of publication by the Company and are subject to change without notice. Past performance should not be taken as an indication or guarantee of future performance, and no representation or warranty, express or implied is made regarding future performance.
A complete description of the risks and uncertainties relating to the Company and its securities can be found in the company's filings with the U.S. Securities and Exchange Commission available for free at www.sec.gov.
Information on this page may relate to penny stocks, which may also be referred to as low-priced stocks. Penny stocks are low-priced shares typically issued by small companies. Penny stocks involve greater than normal risk, they may be less liquid than other stocks (i.e., more difficult to sell), and there may be less reliable information available regarding such stocks. Investors in penny stocks should be prepared for the possibility that they may lose their entire investment.
b2i Digital or its related entities may own securities of the Company.
To comply with Rule 17(b) of the Securities Act of 1933, as amended, b2i Digital must provide full disclosure of all compensation received for investor awareness services provided by the Company.
The Company is a client of b2i Digital. The Company agreed to pay b2i Digital no greater than $100,000 in cash for 12 months of digital marketing consulting and investor awareness services.
The BioRestorative Therapies management team and investor relations team is available to talk to current and potential investors. They're happy to answer your questions and tell you what makes their story unique. Please fill out this form, and we will connect you shortly.
• Directly hear the BioRestorative story
• Ask your questions
• Submit the form below and someone will get in touch with you as soon as possible
Note: Company management or its representative can only discuss and disclose information that is already available in the public domain. They will do their best to clarify such information to the extent permitted by securities law and industry regulations.






A Robust Pipeline for Myriad of Indications
BioRestorative Therapies was founded by scientists and clinicians committed to developing stem cell therapies to address unmet needs in patients with highly prevalent conditions.