Latest Updates
OceanPal Inc.
OceanPal Inc. (Nasdaq: OP) is a global provider of shipping transportation services, specializing in the ownership and operation of dry bulk vessels and product tankers. As of February 13, 2025, the company’s fleet included three Panamax dry bulk vessels and one MR2 tanker vessel, offering flexible transport solutions for both dry and liquid cargoes.
OceanPal works closely with leading international charterers, shipbuilders, and financial institutions, supported by a reputation for operational excellence and experienced management. With no outstanding debt and a strong balance sheet, the company is positioned to capitalize on market opportunities and support the evolving needs of global trade.
Stock Details
Recent News
SEC Filings
OceanPal Inc. At A Glance
OceanPal Inc. provides global shipping transportation services by owning and operating dry bulk vessels and product tankers. As of February 2025, the company’s fleet includes three Panamax dry bulk vessels and one MR2 tanker vessel, offering flexible solutions for transporting dry and liquid cargoes. OceanPal works with leading international charterers, shipbuilders, and financial institutions, supported by partnerships with top technical and commercial managers. With no outstanding debt and a focus on operational efficiency, OceanPal is positioned to respond to shifting market dynamics in global trade. Click below to learn more about OceanPal’s fleet, operations, and business strategy.
Focused Provider of Dry Bulk and Tanker Shipping Services
OceanPal Inc. owns and operates a fleet of three Panamax dry bulk vessels and one MR2 tanker vessel, providing flexible solutions for transporting dry and liquid cargoes. The company’s vessels serve key global trade routes, supporting the movement of essential commodities such as grain, coal, and oil products.
Debt-Free Balance Sheet with Financial Flexibility
Unlike many peers in the shipping sector, OceanPal operates with no outstanding debt, offering unique financial flexibility. This approach allows the company to prioritize operational efficiency, pursue growth opportunities, and manage through market cycles without the burden of significant debt service obligations.
Strong Industry Relationships and Operational Expertise
OceanPal benefits from established relationships with international charterers, shipbuilders, and financial institutions. The company works closely with experienced technical and commercial managers—including Diana Wilhelmsen Management Limited and Anglo-Eastern Shipmanagement—ensuring high safety, regulatory, and operational standards.
Experienced Leadership with Deep Sector Knowledge
OceanPal is led by CEO Robert Perri, CFO Vaso Plousaki, and Chief Corporate Development & Governance Officer Margarita Veniou, who bring decades of combined experience in maritime operations, finance, and corporate governance. Their leadership supports a disciplined strategy focused on asset quality and market adaptability.
Positioned to Navigate a Dynamic Shipping Market
The global shipping market remains influenced by shifting trade patterns, regulatory changes, and evolving demand for dry bulk and energy commodities. OceanPal’s well-maintained fleet, combined with its lean cost structure, positions the company to respond to market changes and chartering opportunities.
Commitment to Operational and Financial Discipline
Through regular vessel inspections, ongoing maintenance, and careful chartering strategies, OceanPal maintains a focus on operational reliability and cost control. The company’s conservative financial posture and emphasis on vessel quality contribute to its ability to generate value over the long term.
Digging Deeper
-
Click to view Analyst CoverageAnalyst Coverage
-
Click to view Addressing Important Unmet NeedsAddressing Important Unmet Needs
-
Click to view Reducing Pill BurdenReducing Pill Burden
-
Click to view Financing the FutureFinancing the Future
-
Click to view PipelinePipeline
-
Click to view Oxylanthanum Carbonate: Innovative Approach to Phosphate ControlOxylanthanum Carbonate: Innovative Approach to Phosphate Control
-
Click to view UNI-494: Focused on MitochondriaUNI-494: Focused on Mitochondria
-
Click to view A Large Addressable MarketA Large Addressable Market
-
Click to view Deftly Navigating the Regulatory MazeDeftly Navigating the Regulatory Maze
-
Click to view Digging into the Science Pt. 1Digging into the Science Pt. 1
-
Click to view Digging into the Science Pt. 2Digging into the Science Pt. 2
-
Click to view Risks & DisclosuresRisks & Disclosures
Analyst Coverage
FIRM |
ANALYST |
|
Brookline Capital Markets |
Kumar Raja |
|
H.C. Wainright & Co. |
Edward Arce |
|
Maxim Group |
Jason McCarthy, Ph.D. |
|
Noble Life Science Partners |
Robert LeBoyer |
|
Piper Sandler |
Yasmeen Rahimi, Ph.D. |
|
Rodman & Renshaw |
Elemer Piros |
|
The Benchmark Company, LLC |
Bruce Jackson |
Unicycive Therapeutics, Inc. is followed by the analysts listed above. Please note that any opinions, estimates, or forecasts regarding Unicycive Therapeutics, Inc.'s performance made by these analysts are theirs alone and do not represent opinions, forecasts, or predictions of Unicycive Therapeutics, Inc., B2i Digital, Inc., or their respective management. Neither Unicycive Therapeutics, Inc. nor B2i Digital, Inc., by its reference above or distribution, imply its endorsement of or concurrence with such information, conclusions, or recommendations.
Addressing Important Unmet Needs
Unicycive Therapeutics is a different type of biotech company. Unlike most firms in this space, Unicycive was built from the ground up in only three years. Additionally, they are focused on diseases of the kidney, a space that has had a shortage of effective and convenient solutions.
Chronic Kidney Disease (CKD) ranges between stages 1 through 5, with 1 being the least severe and 5 being the most. Stage 5 is called End-Stage Renal Disease (ESRD), where kidney function has declined to the point that patients require a kidney transplant or go on long-term dialysis to maintain life. At this late stage of CKD, elevated phosphorus, known as hyperphosphatemia, becomes a serious problem that needs to be addressed. Unfortunately, phosphate control can become a real challenge for CKD patients because, currently, available therapies require a burdensome number of large pills. Unicycive's drug Oxylanthanum Carbonate (OLC), if approved, promises to reduce that burden dramatically.

That phosphate control can become incredibly uncomfortable for ESRD sufferers as the number of pills that are necessary can be a major burden. Unicycive's drug Oxylanthanum Carbonate (OLC) intends to reduce that burden dramatically.
Unicycive's management team and board of directors are steeped in experience, both in the medical industry as well as with drug commercialization. Additionally, their scientific board holds the impressive levels of experience and knowledge necessary to properly guide the firm and solve its most pressing issues.


Source: Company Documents
Reducing Pill Burden
OLC may reduce pill burden volume by more than 4-fold, compared to the most prescribed phospate binder.


Source: Company Documents
For any biotech company looking to build a pipeline and create the next important pharmaceutical, having strong financing in place is critical. You need a sufficient runway to see a pharmaceutical solution from concept through the regulatory process and into the market.
Unicycive recently announced in March 2024 a $50 million private placement, providing significant funding to support its clinical development pipeline. The financing, led by new investors Octagon Capital and Great Point Partners, LLC, with participation from other institutional investors, ensures that Unicycive can advance its lead drug, Oxylanthanum Carbonate (OLC), through the approval process and prepare for a commercial launch.
In addition to this $50 million, Unicycive has access to up to $100 million in committed capital through future warrant exercises. The warrants are linked to significant milestones in the regulatory process, with Tranche A warrants exercisable upon FDA approval for OLC, Tranche B exercisable upon TDAPA approval, and Tranche C exercisable after four quarters of commercial sales. This additional funding commitment underscores the investors' confidence in Unicycive's programs and their potential for success.
Proceeds from the private placement will also support Unicycive's NDA submission with the FDA for approval of OLC for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis. This funding positions Unicycive to navigate regulatory hurdles and successfully bring its innovative treatments to market.
With this robust financial backing, Unicycive is well-equipped to advance both its lead program, OLC, and its secondary program, UNI-494, focused on acute kidney injury, toward clinical and commercial success.

Source: Company Documents
Unicycive's Single-Minded Attention:
Focused on developing new treatment options for renal diseases.

Source: Company Documents
Treating End-Stage Renal Disease (ESRD) demands effective phosphate control to avoid complications such as heart failure. Current solutions often require burdensome and expensive regimens with large pills. Oxylanthanum Carbonate (OLC) is positioning itself as a novel and elegant solution to this problem.
OLC is an advanced phosphate-binding agent using proprietary nanoparticle technology, designed to treat hyperphosphatemia in chronic kidney disease (CKD) patients on dialysis. The nanoparticle technology increases the surface area of active drugs, allowing more medicine in smaller pills that can be swallowed whole, reducing the pill burden.
Unicycive Therapeutics completed a clinical trial with 32 healthy volunteers to study OLC. This study showed that OLC was minimally absorbed into the systemic circulation and was safe and well-tolerated at doses up to 6000 mg/day. The results indicated that OLC significantly reduced urine phosphate excretion and increased fecal phosphate excretion at doses at and above 3000 mg/day, demonstrating its potential effectiveness in managing hyperphosphatemia.

OLC* is a novel investigational phosphate binder for the treatment of hyperphosphatemia in patients with chronic kidney disease on dialysis. Its mechanism of action involves binding to dietary phosphate in the gastrointestinal tract, which is then excreted via the feces, leading to a reduction in serum phosphorus levels. The key features of OLC include:
-
Enhanced surface area due to nanoparticle technology
-
Lower molecular weight
-
Immediate-release tablets
-
Smaller pills
-
Swallowed whole (not chewed)
*OLC is an unapproved investigational new drug being developed under the FDA's 505(b)(2) regulatory procedure. If approved, it will share the same product label and prescribing information as the reference-listed drug Fosrenol (lanthanum carbonate), with the advantage of smaller pills that can be swallowed whole with water, not chewed.
Unicycive has a strong patent portfolio protecting OLC, with a family of U.S. patents and corresponding patents in Canada, Europe, Japan, China, Australia, and other countries.
Both the U.S. and foreign patents were filed in 2011, with a statutory expiration in 2031. This patent protection offers Unicycive the potential to deliver significant shareholder value for years to come.
Unicycive believes mitochondria play a critical role in Acute Kidney Injury (AKI) due to their dual role as the primary source of cellular energy and key regulators of cell death. Mitochondrial damage in AKI can lead to sublethal and lethal injury of kidney tubules, resulting in a loss of renal function.
Disruption in mitochondrial dynamics and compromised membrane integrity can trigger the release of apoptogenic factors, mitochondrial permeability transition (MPT) pores, loss of membrane potential, energetic failure, and reactive oxygen species production, leading to cell injury and death.

.webp?width=1500&height=722&name=featured-banners-Unicycive-2%20(1).webp)
Currently, there are no FDA-approved medicines to treat AKI. Damage to the kidney is often irreversible, requiring renal transplant or lifelong dialysis.
UNI-494 is a novel chemical entity targeting mitochondrial dysfunction, with the potential to address AKI's unmet medical needs. UNI-494 is in preclinical development for the treatment of AKI, derived from a marketed agent, nicorandil, and designed to improve mitochondrial function by blocking the opening of MPTP pores in the inner mitochondrial membrane.

-
UNI-494 is a prodrug of nicorandil with improved properties and an extended patent life.
-
Nicorandil has compelling scientific data supporting the development of UNI-494 for Acute Kidney Disease (AKI) and Chronic Kidney Disease (CKD).
-
Unicycive is focusing on AKI as the initial indication, with CKD as a possible follow-on program.
Unicycive Therapeutics is executing its go-to-market strategy for UNI-494, aiming to advance the drug through preclinical studies and regulatory filing to initiate clinical trials. The company has achieved key milestones, including completing preclinical studies, manufacturing drug supplies for clinical studies, and obtaining MHRA approval to initiate the first human trial. The Phase 1 clinical trial was initiated in Q1 2023, and Unicycive is working toward an FDA IND filing for a Phase 2 proof-of-concept study.
FDA Regulatory Strategy:
-
Confirm prodrug tolerability in animal studies at desired doses.
-
Identify prodrug dose(s) for initial human study and demonstrate conversion of UNI-494 to nicorandil in humans.
-
Seek regulatory clearance to initiate Phase 1 study.
Unique attributes for regulatory approval of UNI-494:
-
Leverage preclinical and clinical data from nicorandil outside the United States with a comparability package.
-
Design a more homogenous AKI patient population, including patients with contrast-induced nephropathy (CIN), where nicorandil has been shown to be efficacious.
Milestones
Animal safety studies completed
Drug supplies for clinical studies manufactured
MHRA approval to initiate first-in-human trial
Phase 1 initiated (Q1 '23)
Ongoing regulatory filing process to support the Phase 2 proof-of-concept (POC) study
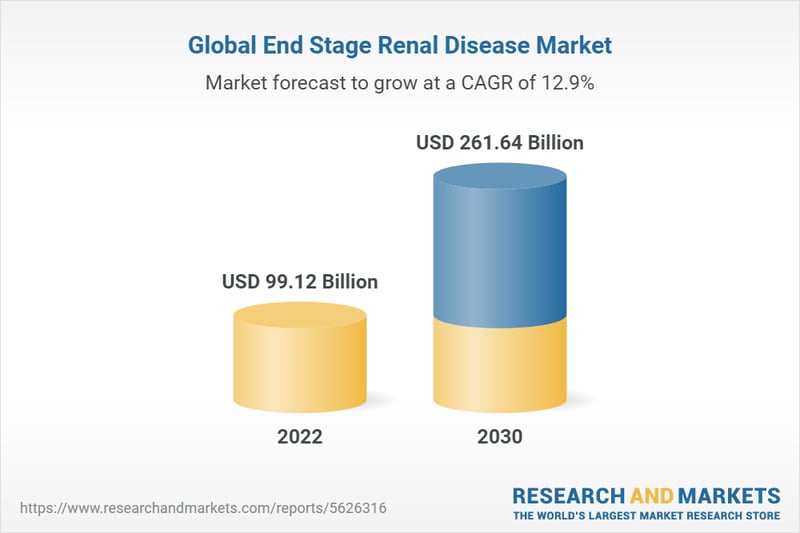
Chronic Kidney Disease is a very significant issue across much of the world. According to Research and Markets, the global end stage renal disease (ESRD) market size is anticipated to reach over $260 billion by 2030, leading to a very robust Compound Annual Growth Rate (CAGR) of 12.9% from 2022 to 2030. An increasing number of patients suffering from kidney failure, the rapid increase in the volume of hospitals & urgent care centers, and growing hospital admission rates are thought to be the catalysts behind this growth. Both of the company's programs have the potential to participate in this market.
Unicycive Therapeutics' lead program, Oxylanthanum Carbonate (OLC), is designed to address hyperphosphatemia, a condition characterized by elevated phosphate levels in patients with chronic kidney disease undergoing dialysis. The global hyperphosphatemia treatment market was estimated to be $2.5 billion in 2021. With a projected Compound Annual Growth Rate (CAGR) of 5.3% between 2021 and 2028, the current market may be close to $3.0 billion, highlighting a significant opportunity for Unicycive's OLC.
Go-to-Market Strategy and Partnerships:
Unicycive's go-to-market strategy involves strategic partnerships to expand the reach and accelerate the commercialization of Oxylanthanum Carbonate. In Q4 2022, Unicycive entered an agreement with Lee's Pharmaceutical (HK) Limited, granting exclusive rights to develop, market, and commercialize OLC in Mainland China, Hong Kong, and certain Asian markets. This agreement provides Unicycive with a strong local partner with deep domain expertise, unlocking significant growth potential in one of the largest markets for kidney disease patients.
As part of the agreement, Unicycive received an upfront payment of $1.0 million and is eligible for royalties on sales and other milestone payments. This strategic partnership enhances Unicycive's presence in Asia, providing a strong platform for future growth.
Unicycive continues to explore additional partnership opportunities in Asia and Europe to further expand its market presence and accelerate the commercialization of Oxylanthanum Carbonate. The company aims to establish a global network of strategic partners to bring innovative treatments to patients with kidney disease worldwide.


Source: Company Documents
As with any company focusing on creating solutions for health issues, navigating the regulatory maze is critical for success. Unicycive has demonstrated a strong ability to do so.
Oxylanthanum Carbonate
The company's lead program, Oxylanthanum Carbonate (OLC), is on a significantly de-risked regulatory pathway for commercialization. In a Type C meeting with the FDA in Q4 2021, the company received clear guidance to file a New Drug Application (NDA). Unicycive is pursuing a 505(b)(2) pathway for U.S. approval, leveraging pre-clinical and clinical data from the approved lanthanum-containing phosphate binder, Fosrenol. This pathway allows the company to skip certain clinical trial phases, streamlining the approval process.
The company conducted a bioequivalence (B.E.) study comparing urinary phosphorus changes between OLC and Fosrenol in healthy volunteers and a 6-month oral toxicity study in mice. The results showed that OLC met all endpoints, indicating its safety and efficacy. Additionally, the company completed standard studies on manufacturability and commercial supply readiness. Unicycive completed the B.E. study with volunteers in Q4 2022 and is on track to file the NDA in mid-2023.
With a one-year review process, potential FDA approval could be anticipated in 2024.
Oxylanthanum Carbonate regulatory strategy and key milestones
FDA Regulatory Strategy
-
505(b)(2) regulatory pathway for the potential U.S. approval of OLC.
-
Leverage pre-clinical and clinical data from Fosrenol to support NDA.
-
Bioequivalence study in healthy volunteers comparing urinary phosphorus changes between OLC and Fosrenol.
-
6-month oral toxicity study in mice.
-
Treatment-emergent adverse events were comparable between the OLC and Fosrenol groups, with no serious adverse events or deaths reported.
Commercial strategy
-
Product positioning, market access, and market shaping activities are ongoing to optimize the value proposition.
-
Pursuing a parallel commercial model to address the concentrated nephrology market, including partnerships with pharmaceutical companies, license and/or distribution agreements with dialysis organizations, and outsourced contract sales organizations.
*OLC is an unapproved investigational new drug being developed under FDA’s 505(b)(2 ) regulatory procedure. If approved, OLC will share the same product label and prescribing information as the approved reference-listed drug (RLD) Fosrenol, with the advantage of smaller, immediate-release tablets that are swallowed whole and not chewed.
UNI-494
Unicycive's other program, UNI-494, is also positioned well for a shorter approval timeframe. As a novel pro-drug of a marketed agent, nicorandil, it uses an established legacy with known MOAs and demonstrated safety and efficacy with its use of nitric oxide. It uses a known and safe chemical linker, and its controlled release of the active drug in plasma may enable once-daily QD dosing.
The clinical development of UNI-494 began in the United Kingdom (U.K.) to expedite the process. The company filed a Clinical Trial Application (CTA) with the Medicines and Healthcare Products Regulatory Agency (MHRA) in the U.K. to initiate a Phase I healthy volunteer study. In addition, Unicycive filed an Investigational New Drug (IND) application with the FDA, and a Phase II proof-of-concept (POC) study is expected in the second half of 2023.
While nothing is guaranteed during this process, Unicycive Therapeutics is taking intelligent steps that could lead to commercialization in a shorter timeframe compared to many other companies in the industry.

Source: Company Documents
Digging into the Science Pt. 1
Unicycive Therapeutics focuses on developing drug candidates for serious kidney diseases. Its lead program, Oxylanthanum Carbonate (OLC), aims to address the challenging problem of uncontrolled hyperphosphatemia with a patient-friendly solution. This serious complication often affects patients undergoing dialysis and can lead to significant health risks if not managed effectively.
Hyperphosphatemia is strongly associated with mortality and hospitalization

Mineral Medbolism, Mortality, and Morbidity in Maintenance Hemodialysis | Geoffrey A. Block, Preston S. Klassen, J. Michael Lazarus, Norma Ofsthun. Edmund G Lowrie and Glenn M. Chertow | JASN August 2004, 15 (8) 2208-2218; DOI: https://journals.lww.com/jasn/abstract/2004/08000/mineral_metabolism,_mortality,_and_morbidity_in.26.aspx
Source: Company Documents
Oxylanthanum Carbonate (OLC) Product Profile
-
Oxylanthanum Carbonate (OLC) is a novel phosphate binder designed to treat hyperphosphatemia in patients with chronic kidney disease (CKD).
-
The mechanism of action involves binding to dietary phosphate in the gastrointestinal (GI) tract, which is then excreted via the feces, resulting in reduced serum phosphate levels.
-
Unicycive's proprietary nanotechnology allows more active medication to be packed into smaller pills, offering greater convenience and efficacy for patients.
-
Key Features of OLC
-
Enhanced surface area due to proprietary nanotechnology
-
Lower molecular weight
-
Immediate-release tablets
-
Smaller pills that are swallowed whole (Not Chewed)
-
Digging into the Science Pt. 2
UNI-494 is Unicycive Therapeutics' other program, targeting Acute Kidney Injury (AKI). This drug is designed to focus on mitochondria—the "powerhouse of the cell"—which play a critical role in energy production and cellular health. Mitochondrial dysfunction can lead to kidney damage, which UNI-494 aims to address by regulating mitochondrial activity.


Source: Company Documents
UNI-494 restores mitochondrial function mechanism of action

-
A hallmark feature of mitochondrial dysfunction is the chronic opening of MPT Pores and the overproduction of Reactive Oxygen Species (ROS)
-
Chronic opening of MPT Pore leads to water and solute influx, injury, and subsequent death
-
UNI-494 is an ATP-sensitive K channel (KATP) activator
-
Binds to SUR2 subunit of KATP channel that, in turn, leads to closing of MPT pores
-
Nicorandil down-regulates the production of ROS
This communication is neither an offer to sell nor a solicitation of an offer to buy, nor a recommendation of any securities of the company mentioned herein.
Unicycive Therapeutics Inc (the “Company”) and its counsel have reviewed the content of this page as well as the accompanying presentation (“Company Presentation”) displayed on this page. To the best of its knowledge, the Company does not believe this content to be misleading or inaccurate in any material respect, nor does it believe there are any material omissions with respect to such content. The Company does not believe the contents of the page or the Company Presentation to contain any non-public material information.
Information and opinions presented in the Company Presentation are provided by the Company, and b2i Digital makes no representation as to their accuracy or completeness. The information contained on this page is not intended to constitute any form of advice, and the information provided is not intended to provide a sufficient basis on which to make an investment decision. It is not investment research, nor does it constitute a research recommendation, as it does not constitute substantive research or analysis. This information is not to be relied upon in substitution for the exercise of independent judgment.
Information, opinions and estimates contained on this page or in the Company Presentation reflect judgments by the Company as of the original date of publication by the Company and are subject to change without notice. Past performance should not be taken as an indication or guarantee of future performance, and no representation or warranty, express or implied is made regarding future performance.
A complete description of the risks and uncertainties relating to the Company and its securities can be found in the company's filings with the U.S. Securities and Exchange Commission available for free at www.sec.gov.
Information on this page may relate to penny stocks, which may also be referred to as low-priced stocks. Penny stocks are low-priced shares typically issued by small companies. Penny stocks involve greater than normal risk, they may be less liquid than other stocks (i.e., more difficult to sell), and there may be less reliable information available regarding such stocks. Investors in penny stocks should be prepared for the possibility that they may lose their entire investment.
B2i Digital or its related entities may own securities of the Company. Specifically, the CEO of B2i Digital owns 22,000 shares of unrestricted UNCY stock as of August 14, 2024.
To comply with Rule 17(b) of the Securities Act of 1933, as amended, B2i Digital must provide full disclosure of all compensation received for investor awareness services provided by the Company.
The Company is a client of B2i Digital. The Company agreed to pay B2i Digital no greater than $100,000 in cash for 12 months of digital marketing consulting and investor awareness services.
-
Click to view Proxy Design & Production ServicesProxy Design & Production Services
-
Click to view Investor Relations & Press DistributionInvestor Relations & Press Distribution
-
Click to view SEC EDGAR FilingsSEC EDGAR Filings
-
Click to view Financial Printing ServicesFinancial Printing Services
-
Click to view Virtual Data Room Services (VDRs)Virtual Data Room Services (VDRs)
-
Click to view iXBRL FilingsiXBRL Filings
-
Click to view Mailing Services & Document Management SolutionsMailing Services & Document Management Solutions
Proxy Design & Production Services
The Nuvo Group’s Proxy Design & Production Services help companies streamline the proxy process by delivering professionally designed, accurate, and easy-to-navigate documents that align with corporate goals and boost shareholder participation.
• 24/7 Production team ensures rapid turnaround times with decades of industry expertise.
• End-to-end support manages the entire process from initial design through filing and printing.
• Comprehensive printing offers solutions ranging from simple black ink to premium custom finishes.
• Brand consistency maintains corporate identity standards across all communication materials.

Investor Relations & Press Distribution
NUVOIR services by The Nuvo Group combine compelling messaging with targeted engagement strategies to help organizations reach the right audience and attract new investors. These services deliver tangible results and measurable returns on investment (ROI) for private companies, public companies, and agencies.
• Data-driven strategies combine industry expertise with analytics to create standout messaging.
• Global distribution press releases reach targeted media outlets, journalists, and analysts.
• Engagement tools include webcast services, IR feeds, real-time alerts, and website hosting.
• Flat-fee pricing helps clients maintain cost controls, manage costs predictably, and stay within budget.

SEC EDGAR Filings
When it comes to SEC EDGAR filings, precision, compliance, and timeliness are non-negotiable. The Nuvo Group ensures that every financial filing meets the highest standards, covering mutual funds, proxy statements, annual reports, prospectuses, and key forms such as 10-Q, 10-K, 8-K, 6-K, 11-K, S-1, DEFM14A, and DEFM14C.
• Compliance excellence with meticulous quality reviews ensures all technical requirements are met.
• Software compatibility, working with clients' platforms, eliminates formatting errors.
• 24/7 Availability and around-the-clock support ensure every deadline is met.
• Our expert team of industry veterans helps clients navigate complex filing requirements.

Financial Printing Services
The Nuvo Group offers comprehensive printing solutions for all essential financial documents, including annual reports, proxy statements, offering memorandums, and investor communications.
• Diverse print options run the gamut from black-and-white reports to premium multi-color finishes.
• Advanced production is done with state-of-the-art offset and digital printing capabilities.
• 24/7 Service ensures on-time delivery for time-sensitive projects.
• Custom binding offers multiple finishing options including spiral, coil, and hardcover formats.

Virtual Data Room Services (VDRs)
The Nuvo Group's Virtual Data Room Services (VDRs) provide secure, intuitive platforms for managing sensitive documents during mergers, acquisitions, and capital raises.
• Intuitive interface enables quick document uploads and organization with minimal user training required.
• Document control allows customized access levels and permissions for viewing, downloading, or editing files.
• Security protocols incorporate encryption, multi-factor authentication, and comprehensive activity tracking.
• 24/7 Availability ensures seamless collaboration across time zones during critical transactions.

iXBRL Filings
The Nuvo Group provides inline eXtensible Business Reporting Language (iXBRL) tagging and submission services, ensuring financial statements comply with SEC requirements while meeting critical deadlines.
• Quality assurance maintains precise tagging and alignment with SEC taxonomies through rigorous review processes.
• System integration works within clients' existing financial platforms to maintain formatting consistency.
• Real-time updates enable immediate corrections and adjustments throughout the submission process.
• Expert solutions handle complex financial data across annual reports, proxy statements, and mutual fund filings.

Mailing Services & Document Management Solutions
The Nuvo Group streamlines the delivery of financial communications through comprehensive mailing and document management solutions, from annual reports to shareholder notices.
• Complete mailing handles everything from binding and inserting to domestic and international delivery.
• Secure management provides encrypted portals for document uploads and processing.
• Status tracking offers real-time updates and reporting throughout the delivery process.
• Flexible options accommodate both large-volume mailings and specialized communications.
Video Library
Vycor Medical Corporate Video Overview
Vycor Medical, Inc. is dedicated to providing the medical community with innovative and superior neurosurgical and neurotherapeutic solutions. Vycor Medical designs, develops, and markets medical devices for neurosurgery. ViewSite Brain Access System (VBAS) is a clear cylindrical disposable set of devices of different sizes that neurosurgeons use to provide a surgical corridor to access sites within the brain, such as tumors. This Corporate Video provides testimonials from Neurosurgeons, our VBAS product being used during surgery, VBAS product information, and Vycor Medical company information.
NovaVision Vision Restoration Therapy
NovaVision’s mission is to improve the vision of patients with neurological visual impairments and enhance the quality of life for our patients and their families. The company provides a portfolio of FDA-registered therapy and diagnostic products for vision disorders resulting from a stroke or brain injury. NovaVision Vision Restoration Therapies are evidence-based and supported by decades of scientific research and clinical studies.
Important Resources
Including an At-A-Glance PDF, a document tailored to those who just want quick and summarized information.
Management Team

ROBERT PERRI
ROBERT PERRI, CFA
Chief Executive Officer
Robert Perri has served as the Chief Executive Officer of the Company since February 2023. From June 2021 to December 2022, Mr. Perri worked in the Finance Department of Costamare Inc., a publicly traded company. From November 2016 to June 2021, Mr. Perri was the Chief Financial Officer of TMS Cardiff Gas, Ltd., a private shipping company. Mr. Perri has served as a Director of Kalon Acquisition Group since 2019. In addition, Mr. Perri has spent ten years in equity research for several investment banks covering various industries, including shipping, technology, and IT services. Mr. Perri is a member of the Chartered Financial Analyst (CFA) Institute and a CFA charterholder. Mr. Perri received his Bachelor of Science degree in Accounting and Finance from Drexel University in 1995 and received his MBA with a focus on finance and banking from SDA Bocconi in 1999.

VASILIKI PLOUSAKI
VASILIKI (VASO) PLOUSAKI
Chief Financial Officer
Vasiliki Plousaki has served as the Chief Financial Officer of the Company since April 2023. Mrs. Plousaki has also served as the Chief Accounting Officer of the Company from June 2021 to April 2023, during which time she has been responsible for all financial reporting requirements. From 2020 to June 2021, she was employed by Drew Marine, a global maritime company, as EMEA Regional Controller. In 2011, Mrs. Plousaki joined the Athens branch of Ernst and Young (Hellas), where she progressed to Senior Manager and served as an external auditor specializing in audits of US-listed shipping companies until 2020. Mrs. Plousaki is a member of the Association of Chartered Certified Accountants (ACCA), holds a Bachelor’s degree in Finance from the University of Athens and a Master’s degree in Auditing and Accounting from the University of Athens and the Greek Institute of Chartered Accountants.

MARGARITA VENIOU
MARGARITA VENIOU
Chief Corporate Development & Governance Officer and Secretary
Margarita Veniou has served as the Chief Corporate Development and Governance Officer of the Company since November 2021 and has also served as the Secretary of the Company since April 2023. She has been responsible for the implementation and supervision of general corporate matters, including the development of strategic plans. Ms. Veniou also serves as Chief Corporate Development, Governance & Communications Officer of Diana Shipping Inc. (NYSE: DSX) and Corporate Development, Governance & Communications Manager of Diana Shipping Services S.A., a ship management company, since July 2022. From September 2004 to June 2022, she worked for the same companies, holding various positions as an Associate, Officer, and Manager in the fields of corporate planning and governance. Ms. Veniou held the position of Corporate Planning & Governance Officer from January 2010 to February 2020 in Performance Shipping Inc., a US-listed company. She is also the General Manager of Steamship Shipbroking Enterprises Inc. since April 2014. She is a member of WISTA Hellas and holds a bachelor΄s degree in Maritime Studies and a master΄s degree in Maritime Economics & Policy from the University of Piraeus. She completed the Sustainability Leadership and Corporate Responsibility course at the London Business School. She has obtained certification in Shipping Derivatives from the Athens University of Economics and Business, and she is an ISO 14001 certified by Lloyd’s Register.
OceanPal Inc.'s management has decades of industry experience.
The team brings deep expertise in global shipping, vessel operations, and corporate strategy, focusing on delivering high-quality transport solutions and maintaining strong industry relationships.
The OceanPal Inc. executive leadership regularly updates investors with company news. Please fill out this form to receive the latest information.
Note: The company can only disclose information that is shared in the public domain through press releases, SEC filings, and other public forums. As securities law and industry regulations require, such information will always be shared with all investors simultaneously.